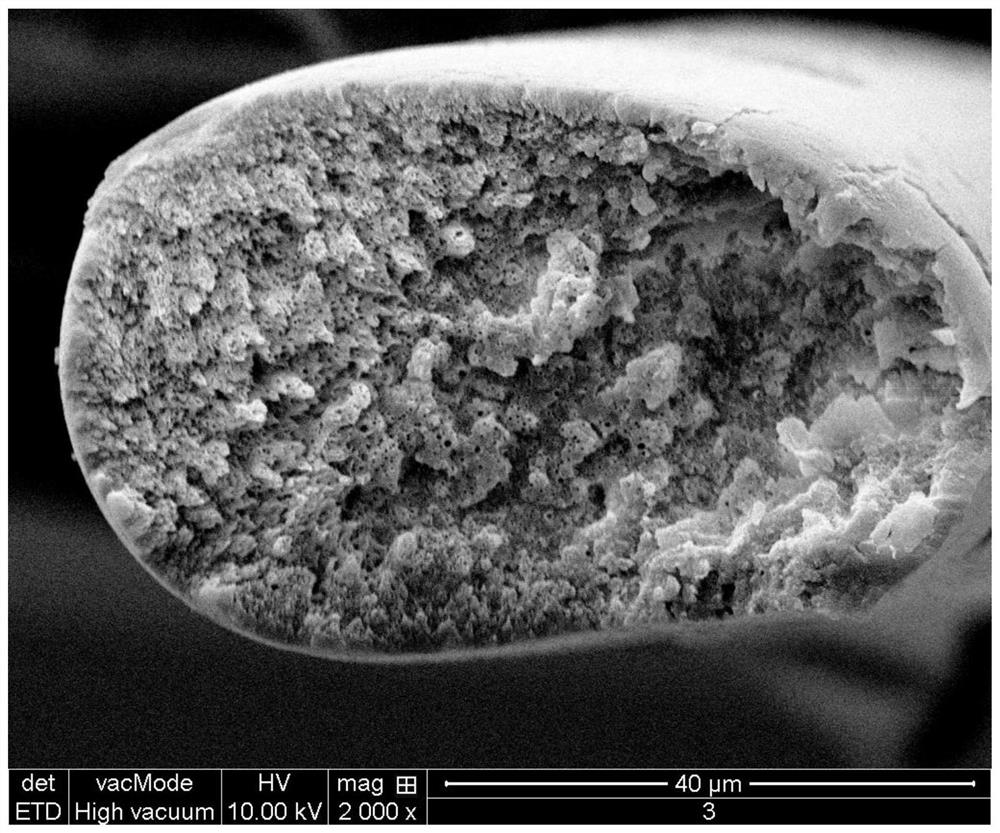
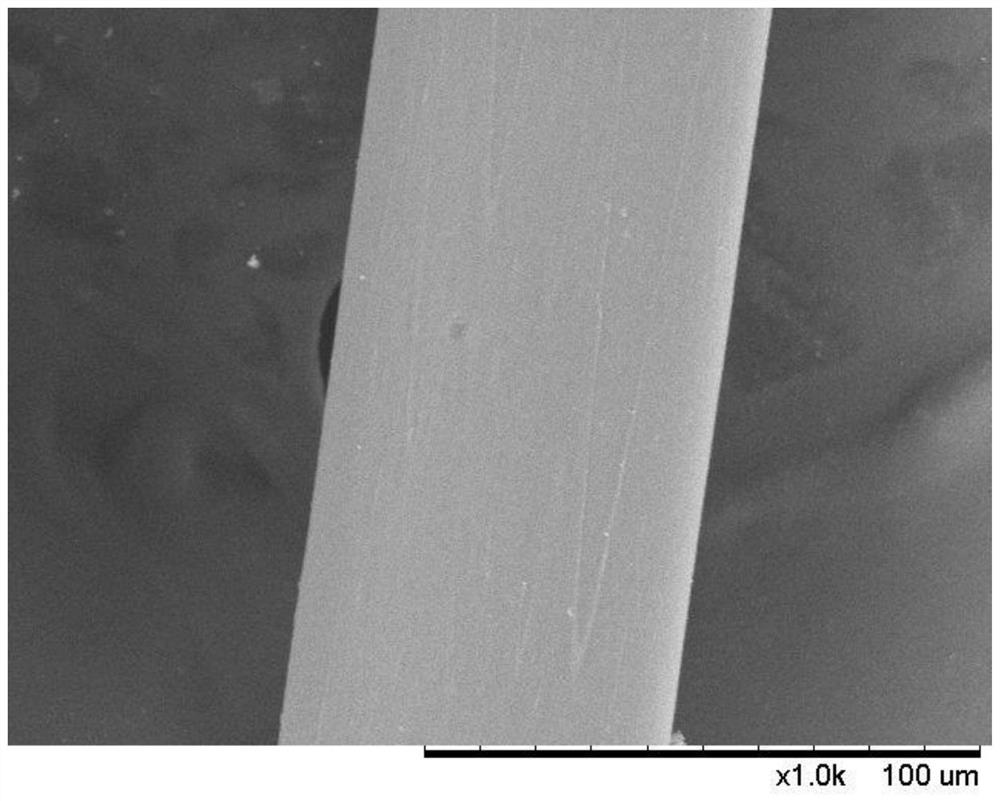
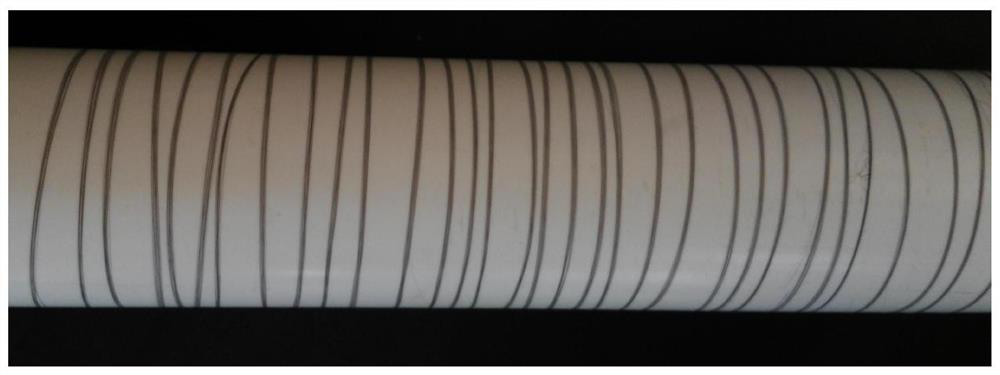
Composite drug-loaded fiber for absorbable surgical suture
A surgical suture and fiber technology, which is applied in the direction of surgery, fiber chemical characteristics, chemical post-treatment of synthetic polymer artificial filaments, etc., can solve the problem of insufficient strength of natural absorbable sutures, few varieties of absorbable sutures, hidden dangers of biological safety, etc. problems, to achieve excellent biocompatibility, excellent tensile properties, and good mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: An anti-inflammatory composite drug-loaded fiber for absorbable sutures—collagen / PLCL / graphene oxide composite drug-loaded fiber of alginic acid / carboxylated chitosan / ketoprofen coating, referred to as : SA-CCTS-KET@COL-PLCL-GO
[0030] The main material is composed of biodegradable natural biomass in vivo - type I collagen (COL) and chemically synthesized biodegradable polymer - polycaprolactone copolymer (PLCL). The reinforced and toughened material is Nano carbon material - graphene oxide (GO), the porogen is a material or agent with good biocompatibility - vitamin E acetate (VEA), curcumin (Cur), the coating material is made of sodium alginate (SA) and carboxylated chitosan (CCTS), and the anti-inflammatory drug contained in the coating material is ketoprofen (KET). The above main materials, reinforcing materials, porogens, coating materials, and anti-inflammatory drugs are compounded and combined with wet spinning, coating and in-situ generation process...
Embodiment 2
[0036] Example 2: An antibacterial composite drug-loaded fiber for absorbable sutures—collagen / PLGA / graphene oxide composite drug-loaded fiber coated with alginic acid / chloramphenicol, referred to as: SA-CAP@COL- PLGA-GO
[0037] The main material is composed of biodegradable natural biomass in vivo - type I collagen (COL) and chemically synthesized biodegradable polymer - polylactic acid copolymer (PLGA), and the reinforced and toughened material is nano-carbon material ——Graphene oxide (GO), the porogen is a material or agent with good biocompatibility—vitamin E acetate (VEA), curcumin (Cur), the coating material is made of sodium alginate (SA) , the antibacterial drug contained in the coating material is chloramphenicol (CAP). The above main materials, reinforcing materials, porogens, coating materials, and antibacterial drugs are compounded and combined with wet spinning, coating, and in-situ generation processes to prepare composite drug-loaded materials that can be used...
Embodiment 3
[0043] Example 3: An anticoagulant composite drug-loaded fiber for absorbable sutures—carboxylated chitosan / heparin-coated silk fibroin / PLGA / single-walled carbon nanotube composite drug-loaded fiber, referred to as: CCTS-UFH@Fibroin-PLGA-SWNT
[0044]The main material is composed of biodegradable natural biomass in vivo - silk fibroin (Fibroin) and chemically synthesized biodegradable polymer - polylactic acid copolymer (PLGA), and the reinforced and toughened material is nano carbon material - —Single-walled carbon nanotubes (SWNT), the porogen is a material with good biocompatibility—polyethylene glycol (PEG), and the coating material is carboxylated chitosan (CCTS). The loaded anticoagulant drug is heparin (UFH). The above main materials, reinforcing materials, porogens, coating materials, and anticoagulant drugs are compounded and combined with wet spinning, coating, and in-situ generation processes to prepare a composite material that can be used for absorbable surgical ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fiber diameter | aaaaa | aaaaa |
| Internal aperture | aaaaa | aaaaa |
| Fiber diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com