Application of long-acting low-addiction compound in preparation of medicine
A compound and drug technology, applied in long-acting and low-addiction compounds, in the application field of diseases, can solve problems such as limiting the long-acting effect of depression and adverse physical and mental effects of patients, and achieve the effect of long drug effect time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1: the synthesis of (2R,6R)-6-hydroxy demethyl ketamine (HNK)
[0088]
[0089] Step 1: In a two-neck bottle or a three-necked bottle, add 50 grams of magnesium powder, and slowly add a mixed solution of 119.2 grams of bromocyclopentane and THF dropwise after initiation. After the dropwise addition, reflux for 2-4 hours to obtain 1.6mol / L Cyclopentyl Grignard Reagent. Add 840 mg of CuBr to the mixture of THF and o-chlorobenzonitrile (50.0 g), and add cyclopentyl Grignard reagent (1.6 mol / L, 280 ml) dropwise under ice-bath conditions. Reflux for 1 hour after the dropwise addition, cool to room temperature, add 100ml of water, then add 200ml of 15% dilute sulfuric acid solution, stir overnight, spin dry THF, extract with EA, dry, and pass through a silica gel column to obtain compound A 2-chlorophenyl ring Amyl ketone 60g, yield 80%.
[0090] Step 2: According to the reported method (Bioorganic & Medicinal Chemistry 2013, 12, 5098), 20 g (96 mmol) of compo...
Embodiment 2
[0098] Embodiment 2: the synthesis of compound I5
[0099] Compound G (170mg, 0.5mmol) was dissolved in 3mL dry THF, dry triethylamine (0.28mL, 2mmol) was added, and then benzoyl chloride (117uL, 1mmol) was added under ice-bath conditions, and then slowly Rise to room temperature, stir overnight, then add sodium bicarbonate solution, extract with EA, spin the solvent and dry in vacuo, pass through a silica gel column to obtain compound H 4 N-Boc-(2R,6R)-6-benzoyloxydesmethylketamine 184mg, yield 85%. 1 H NMR (400MHz, CDCl 3 ): δ8.22-8.19(m, 2H), 7.91(m, 1H), 7.69-7.64(m, 1H), 7.56-7.45(m, 4H), 6.75(br, 1H), 5.54-5.50(m ,1H), 4.00(m,1H), 2.54-2.52(m,1H), 2.14-1.93(m,3H), 1.86(m,1H), 1.41(m,9H). 13 C NMR (100MHz, CDCl 3 ): δ202.2, 164.9, 153.3, 133.2, 131.4, 131.1, 129.9, 129.7, 129.5, 128.3, 128.0, 126.1, 79.2, 73.8, 67.2, 36.6, 34.8, 28.2, 19.0
[0100] Compound H 4 (180 mg) was dissolved in 3 mL of dry THF, gaseous HCl was introduced to saturation at room temperature a...
Embodiment 3
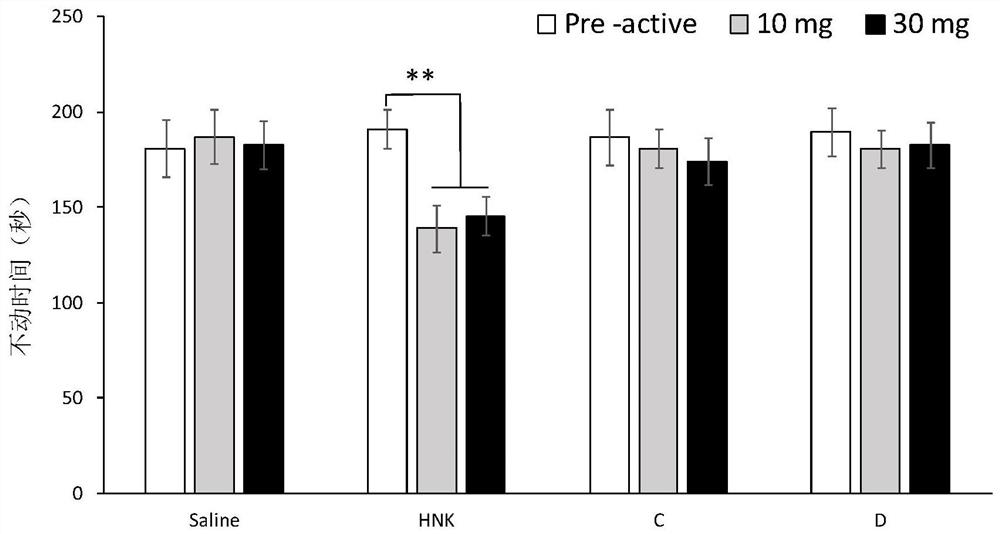
[0103] Embodiment 3: the synthesis of compound C and D
[0104]
[0105] Compounds C and D were prepared by the following preparation methods.
[0106]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com