Synthetic method for metallization of platinum carbene phosphorescent material
A technology for phosphorescent materials and synthesis methods, applied in the directions of luminescent materials, platinum-based organic compounds, chemical instruments and methods, etc., can solve the problems of waste of precious raw materials, low reaction yield and high cost, avoid the risk of explosion and improve the reaction Condition, high safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Platinum (II) complex PtON5N-tt can be synthesized according to the following route:
[0021]
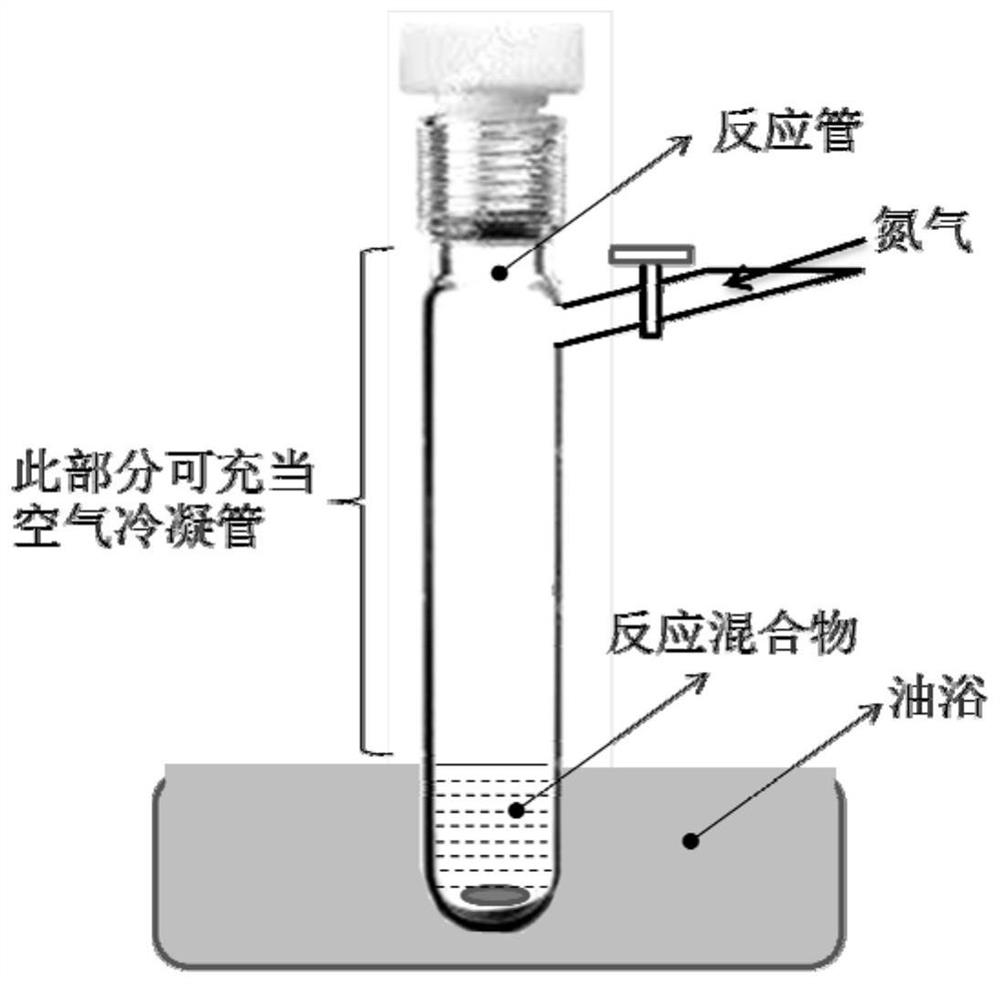
[0022] into a reaction tube with a magnetic stirrer (such as figure 1 ) were sequentially added ligand LON5N-tt (1.69g, 2.33mmol, 1.0eq), (1,5-cyclooctadiene)platinum dichloride (915mg, 2.45mmol, 1.05eq) and sodium acetate (573mg, 6.99mmol , 3.0 equivalents), and then the nitrogen was replaced three times, and diethylene glycol dimethyl ether (64 mL) was added under the protection of nitrogen. Subsequently, the mixture was stirred and reacted in an oil bath at 95-125° C. for 3 days, cooled to room temperature, and the solvent was distilled off under reduced pressure to obtain a crude product. The obtained crude product was separated and purified by silica gel chromatography. The eluent: petroleum ether / dichloromethane volume ratio was 10:1-2:1, and 1.21 g of the product PtON5N-tt yellow solid was obtained with a yield of 67%. 1 H NMR (500MHz, DMSO-d 6):δ1....
Embodiment 2
[0024] Embodiment 2: Platinum (II) complex PtON5N-tt can be synthesized according to the following route:
[0025]
[0026] Add ligand LON5N-tt (3.40g, 4.68mmol, 1.0 equivalent), (1,5-cyclooctadiene) platinum dichloride (1.84g, 4.92mmol, 1.05 equivalent) and sodium acetate (1.15g, 14.04mmol, 3.0 equivalent), then the nitrogen was replaced three times, and diethylene glycol dimethyl ether (68mL) was added under the protection of nitrogen. Subsequently, the mixture was stirred and reacted in an oil bath at 100-130° C. for 3 days, cooled to room temperature, and the solvent was distilled off under reduced pressure to obtain a crude product. The obtained crude product was separated and purified by silica gel column chromatography. The volume ratio of eluent: petroleum ether / dichloromethane was 10:1-2:1, and 2.58 g of the product PtON5N-tt was obtained as a yellow solid with a yield of 71%. 1 H NMR characterization is consistent with Example 1.
Embodiment 3
[0027] Embodiment 3: Platinum (II) complex PtON5N-tt can be synthesized according to the following route:
[0028]
[0029] Add ligand LON5N-tt (100mg, 0.14mmol, 1.0eq), (1,5-cyclooctadiene)platinum dichloride (54mg, 0.144mmol, 1.05eq) sequentially into a reaction tube with a magnetic stirring rotor and sodium acetate (34mg, 0.41mmol, 3.0eq), then pumped nitrogen three times, and added diethylene glycol diethyl ether (15mL) under nitrogen protection. Subsequently, the mixture was stirred and reacted in an oil bath at 100-130° C. for 3 days, cooled to room temperature, and the solvent was distilled off under reduced pressure to obtain a crude product. The obtained crude product was separated and purified by silica gel chromatography. The volume ratio of eluent: petroleum ether / dichloromethane was 10:1-2:1, and 73 mg of the product PtON5N-tt yellow solid was obtained with a yield of 68%. 1 H NMR characterization is consistent with Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Flash point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com