Lipid nanoparticle preparation and application thereof
A technology of lipid nanoparticles and preparations, which can be used in medical preparations without active ingredients, medical preparations containing active ingredients, DNA/RNA vaccination, etc., can solve problems such as limited application space, and achieve good in vitro transfection The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1 Lipid nanoparticle multi-component production procedure
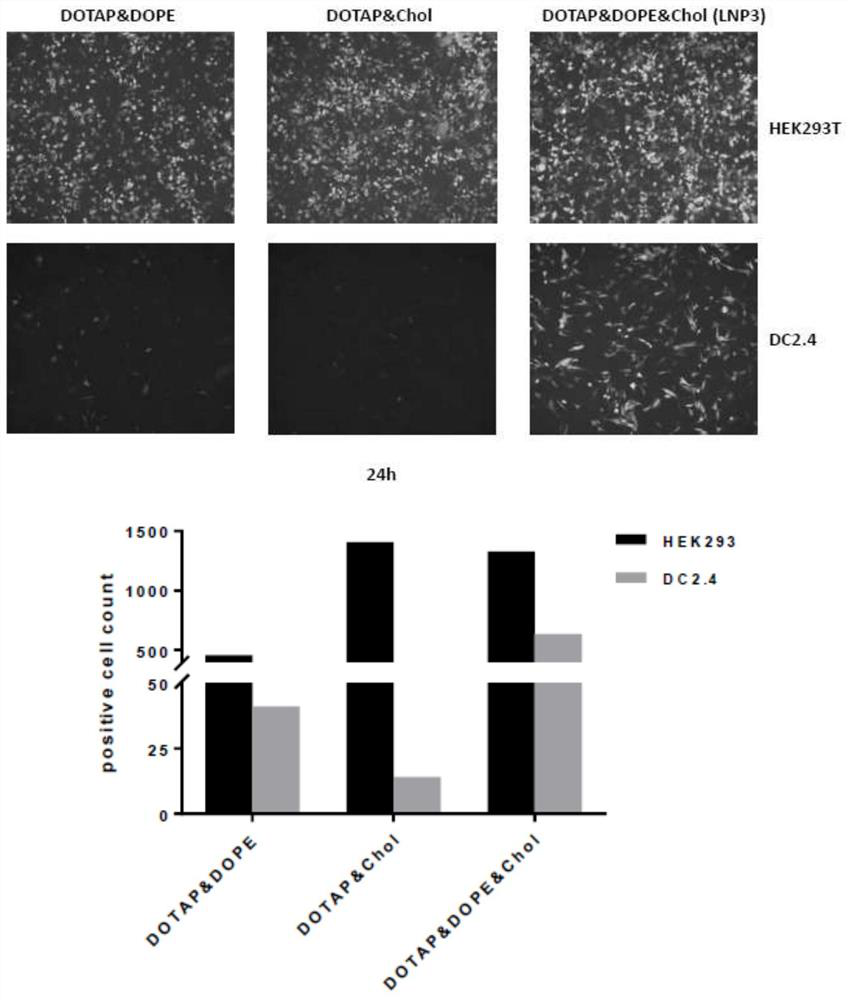
[0081] First, DOTAP, DOPE and cholesterol were prepared into 10 mg / ml solutions in chloroform respectively. According to the molar ratio of DOTAP:DOPE:cholesterol=40:10:48, the above three solutions were mixed uniformly in a round bottom flask. Rotary evaporation under vacuum at 37°C for 1 h formed a film-like solid. Then add DPEC water to prepare a suspension with a concentration of 1 mg / ml. Sonicate for 1.5h, then overnight at 4°C, and sonicate again for 1h the next day to ensure full dissolution. First filter three times with a 0.45um PES filter, then filter three times with a 0.22μm PES filter, and finally filter three times with a 0.1um PES pinhole filter, so as to obtain unloaded nano lipids whose particle size meets the experimental requirements.
[0082] The concentration of the prepared unloaded nano-lipid is 1 mg / ml, and the unloaded nano-lipid and mRNA are prepared according to the char...
Embodiment 2
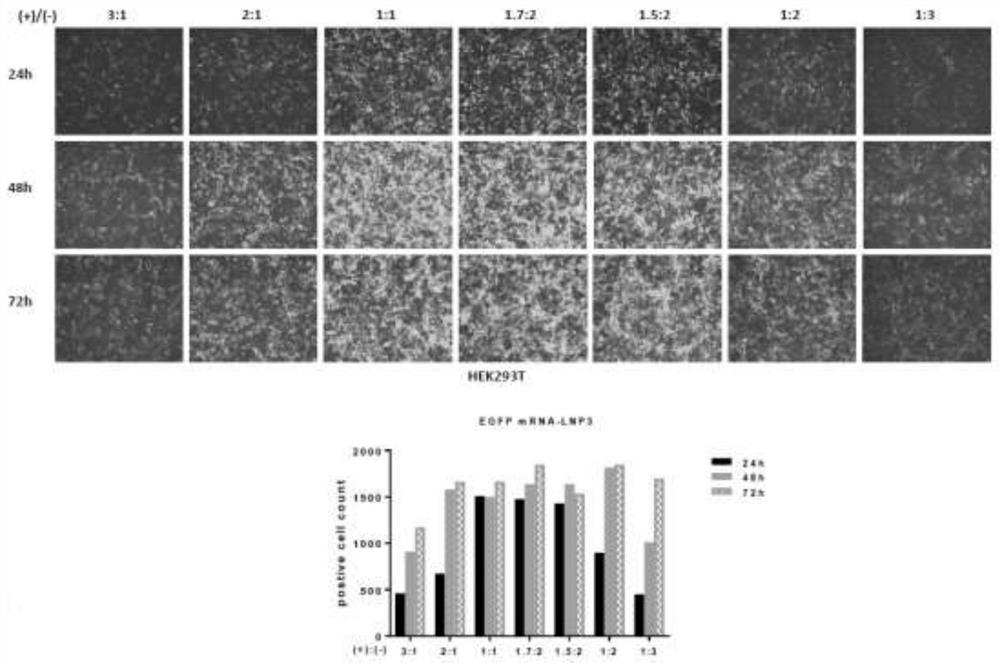
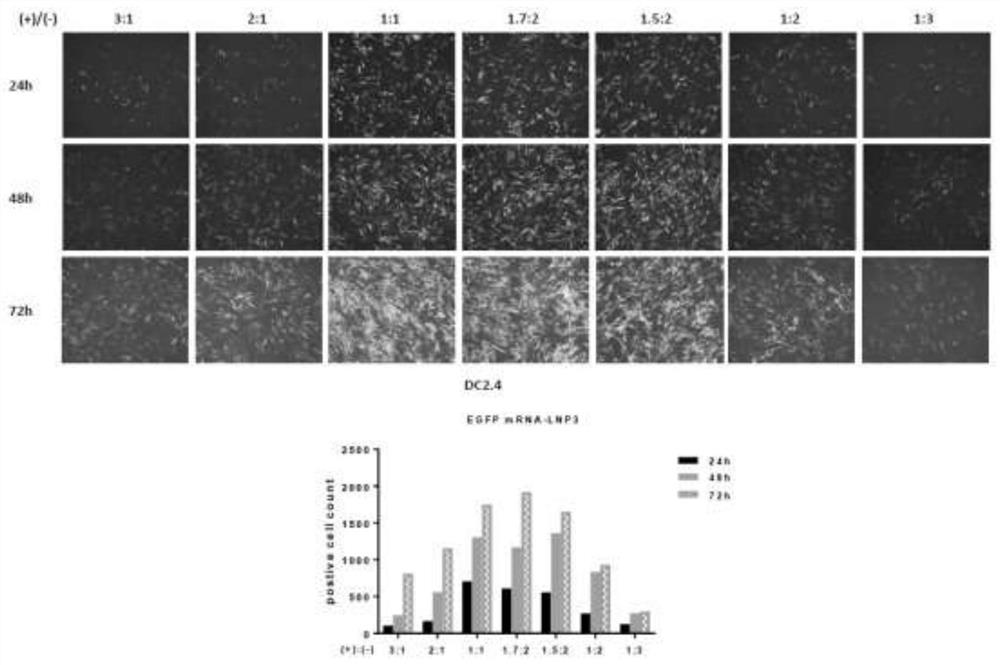
[0084] Example 2 explores the optimal ratio of nano-lipid and mRNA combination to obtain the best transfection effect
[0085] The charge carried by the cationic lipid in the nano-lipid, that is, the positive charge, and the charge carried by the phosphate group in the mRNA, that is, the negative charge, are converted so that the molar ratio of the positive charge to the negative charge (i.e. the quantity ratio) is 3:1, 2: 1, 1:1, 1.7:2, 1.5:2, 1:2 and 1:3. The specific experiment is to use HEK293T and DC2.4 cells as the research objects respectively. First, a certain number of HEK293T and DC2.4 cells are transferred to a 24-well culture plate for culture. Transfection was started when the cell number reached 50-60% by observation under an inverted phase-contrast microscope. Transfect according to the amount of transfection 1ug EGFP mRNA per well, at this time, the molar ratio of positive charge to negative charge (that is, the number ratio) is 3:1, 2:1, 1:1, 1.7:2, 1.5:2,1 ...
Embodiment 3
[0088] Example 3 Cell experiments assess the impact of clinically used injections on the transfection efficiency of preparations
[0089] The purpose of this experiment is to select the most suitable clinical commonly used injection, ensure that the preparation can exist stably in the injection and can effectively transfect cells, and at the same time ensure that the injection can be used in mammals without causing adverse reactions. Currently known injections commonly used clinically include physiological saline, sodium bicarbonate injection, sodium lactate Ringer injection and sodium potassium magnesium calcium glucose injection. In the experiment, normal saline, PBS (phosphate buffer saline), sodium bicarbonate injection, Opti-MEM, lactated Ringer injection and sodium potassium magnesium calcium glucose injection were used as transfection reagents to prepare EGFP mRNA-LNP3. The specific operation is to first transfer a certain number of HEK293T cells to a 24-well plate for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com