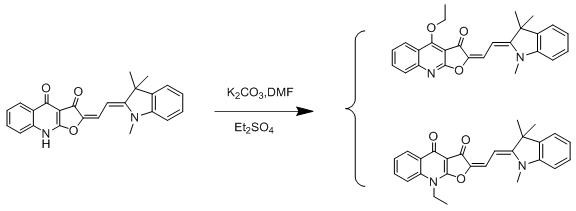
Pair of isomers, and preparation method and application thereof
An isomer, a pair of technology, applied in organic chemistry methods, chemical instruments and methods, analytical materials, etc., can solve problems such as peripheral system toxicity and side effects, and achieve simple post-processing methods, high yields, and fluorescent properties. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Isomers (I) and (II), such as figure 1 As shown, its synthesis steps are as follows:
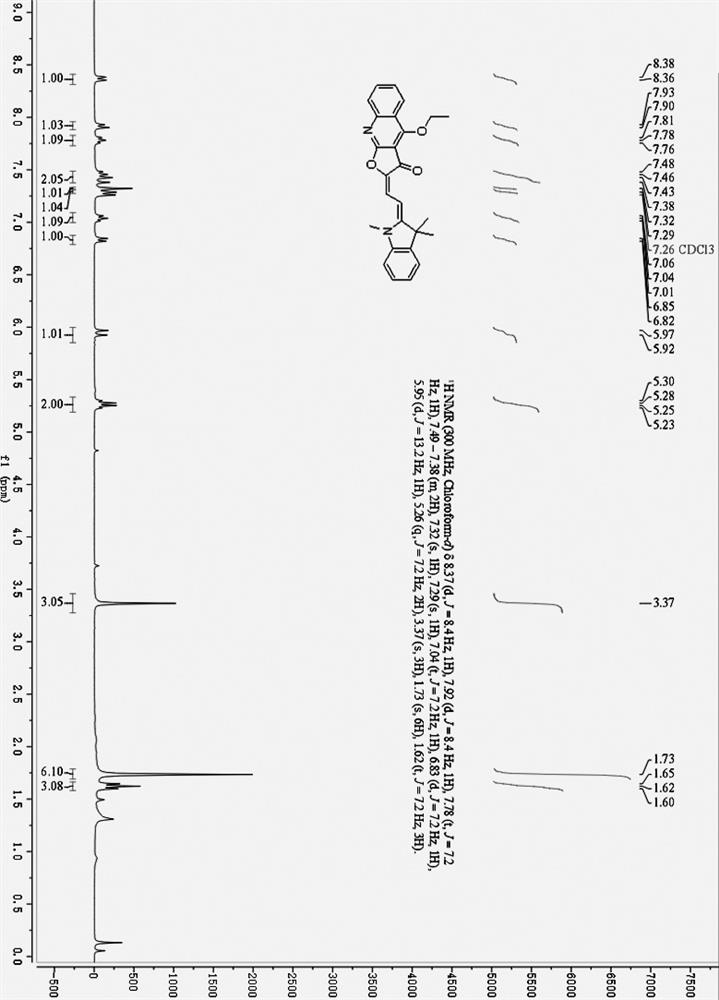
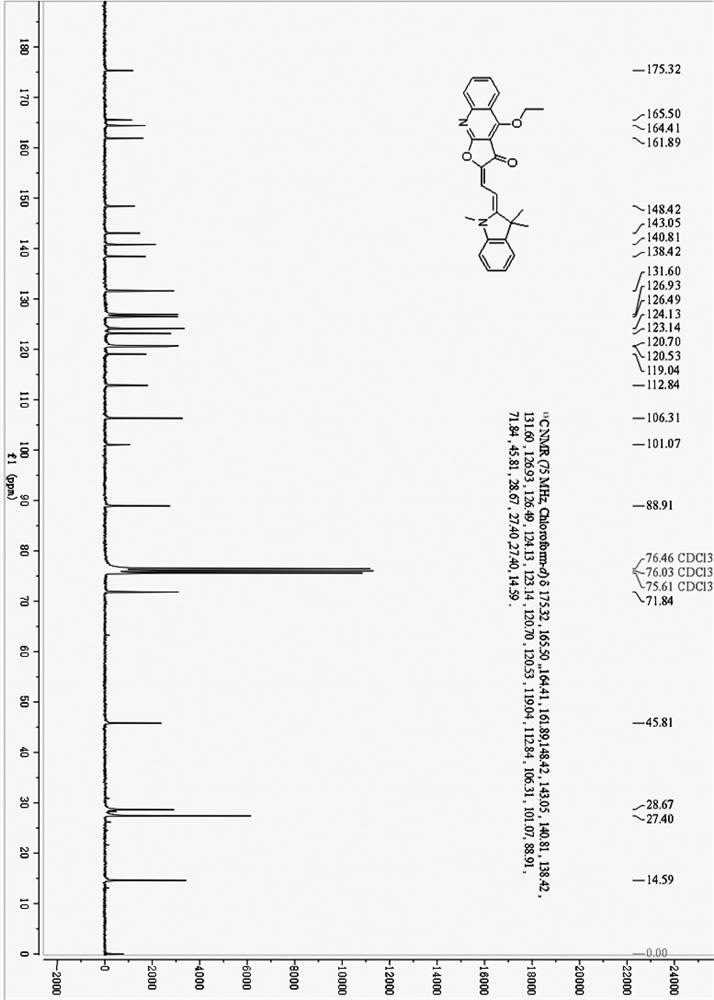
[0040] (E)-2-(2-((Z)-1,3,3-Trimethylindole-2-ethylene)ethylene)furo[2,3-b]quinoline-3, 4(2H,9H)-diketone (384 mg, 1.0 mmol) and anhydrous K 2 CO 3 (207 mg, 1.5 mmol) was added to 30 mL of anhydrous DMF, and 10 mL of a DMF solution containing diethyl sulfate (231 mg, 1.5 mmol) was added dropwise with a constant pressure dropping funnel under stirring. After the dropwise addition was completed, the temperature was raised to 60°C and the reaction was stirred for 30 minutes. After the reaction was completed, the filtrate was obtained by filtration, and the filter cake was washed three times with chloroform, 10 mL each time. The combined solutions were evaporated to dryness, 30 mL of ice water was added, and extracted three times with 30 mL of chloroform. The organic layers were combined, dried over anhydrous sodium sulfate, evaporated to dryness and purified by column chromatography....
Embodiment 2
[0045] The synthesis steps of isomers (I) and (II) of this embodiment are as follows:
[0046] (E)-2-(2-((Z)-1,3,3-Trimethylindole-2-ethylene)ethylene)furo[2,3-b]quinoline-3, 4(2H,9H)-diketone (384 mg, 1.0 mmol) and anhydrous Na 2 CO 3 (318 mg, 3.0 mmol) was added to 30 mL of acetonitrile, and 10 mL of acetonitrile solution containing diethyl sulfate (231 mg, 1.5 mmol) was added dropwise with stirring. After the dropwise addition, the temperature was raised to 70°C and stirred for 30 minutes. After the reaction was completed, the reaction solution was filtered, and the filter cake was washed three times with chloroform, 20 mL each time. Combine the filtrates, evaporate the solvent to dryness, add 30 mL of ice water, extract three times with 30 mL of chloroform, combine the organic layers, dry over anhydrous sodium sulfate, evaporate to dryness and purify by column chromatography. The eluent was petroleum ether / ethyl acetate=2:1 (volume ratio), and 196 mg of isomer (I) was o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com