A kind of synthetic method of tert-butyl hydroquinone
A technology of tert-butyl hydroquinone and a synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problem of uninspected catalyst recycling activity, high process cost, high environmental protection pressure, etc. problems, to achieve the effect of green and clean production process, low process cost and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Weigh 3.0g of hydroquinone, 0.6g of catalyst sulfamic acid, 12.0g of xylene, and 4.0g of tert-butanol in a heatable pressure-resistant reactor, heat the oil bath to 150°C and react for 4 hours;
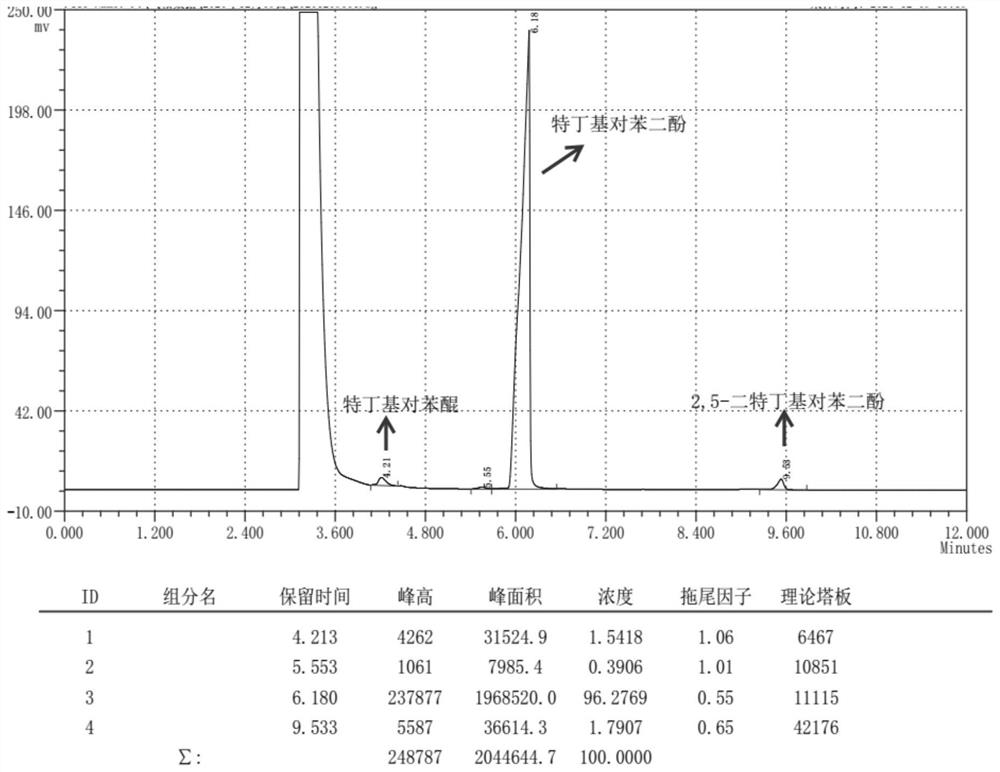
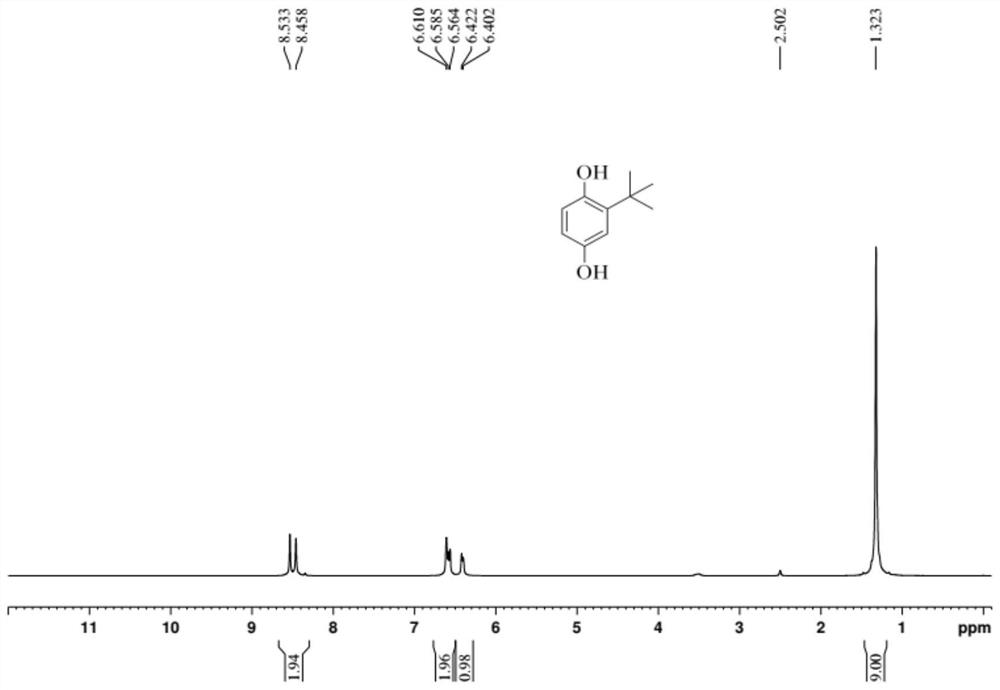
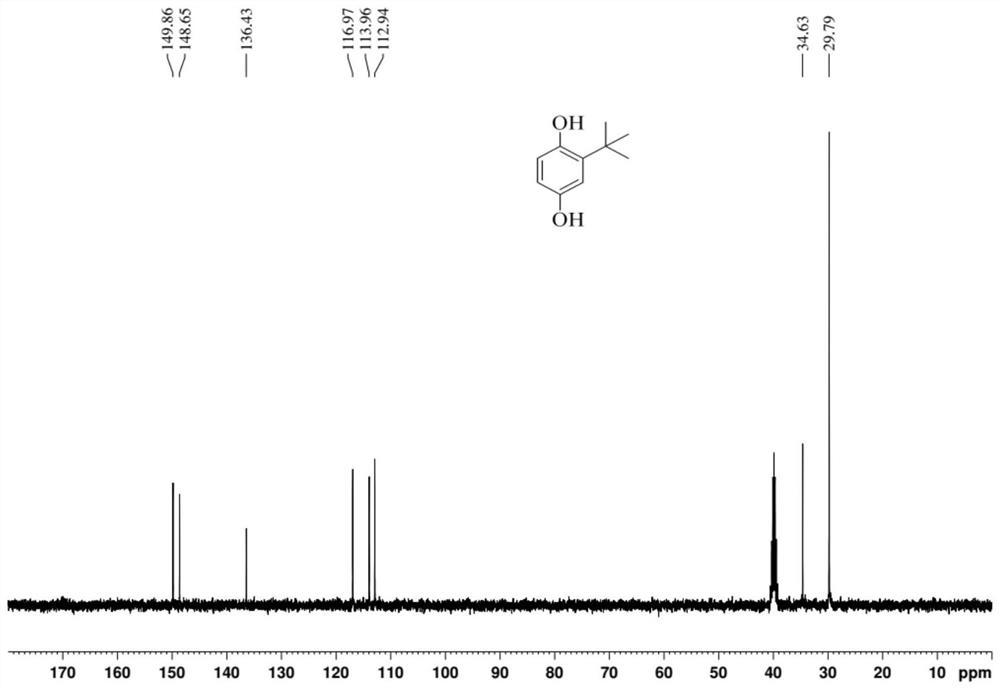
[0032] (2) add ethanol to dissolve crude product after reaction finishes cooling, leave standstill and separate catalyst with Buchner funnel suction filtration, gained filtrate carries out gas chromatography analysis and detection with internal standard method, and wherein the transformation rate of raw material hydroquinone is 86.4%, The yield of the target product tert-butylhydroquinone was 57.8%, the yield of the side-reactant 2,5-di-tert-butylphenol was 17.2%, and the rest were a small amount of ethers and quinones. After the filtrate is desolvated, the product tert-butylhydroquinone can be obtained by washing or recrystallization.
Embodiment 2
[0034] (1) Weigh 3.0g hydroquinone, 0.6g catalyst sulfamic acid, 12.0g toluene, 3.0g tert-butanol, 1.0g 1,4-dioxane in a heatable pressure reactor, oil bath After heating to 120°C, react for 12 hours;
[0035] (2) Add ethanol to dissolve crude product after reaction finishes cooling, leave standstill and separate catalyst with Buchner funnel suction filtration, gained filtrate carries out gas chromatography analysis and detection with internal standard method, and wherein the transformation rate of raw material hydroquinone is 86.7%, The yield of the target product tert-butylhydroquinone was 55.2%, the yield of the by-product 2,5-di-tert-butylphenol was 4.6%, and the rest were a small amount of ethers and quinones. After the filtrate is precipitated, the TBHQ product can be obtained by washing or recrystallization.
Embodiment 3
[0037] (1) Weigh 3.0g hydroquinone, 0.6g catalyst sulfamic acid, 12.0g toluene, 3.0g tert-butanol, 1.0g 1,4-dioxane in a heatable pressure reactor, oil bath After heating to 135°C, react for 4 hours;
[0038] (2) Add ethanol to dissolve crude product after reaction finishes cooling, leave standstill and separate catalyst with Buchner funnel suction filtration, gained filtrate carries out gas chromatography analysis and detection with internal standard method, wherein the conversion rate of raw material hydroquinone is 73.9%, The yield of target product tert-butylhydroquinone was 48.1%, the yield of side-reactant 2,5-di-tert-butylphenol was 9.2%, and the rest were a small amount of ethers and quinones. After the filtrate is precipitated, the TBHQ product can be obtained by washing or recrystallization.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com