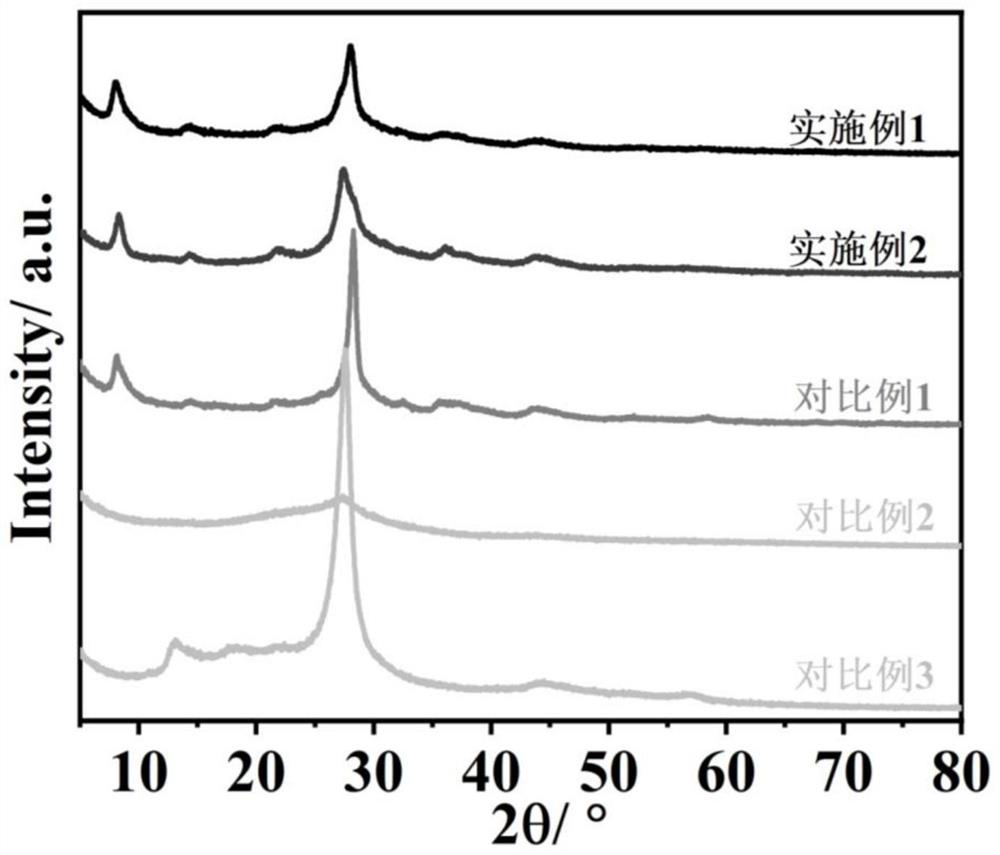
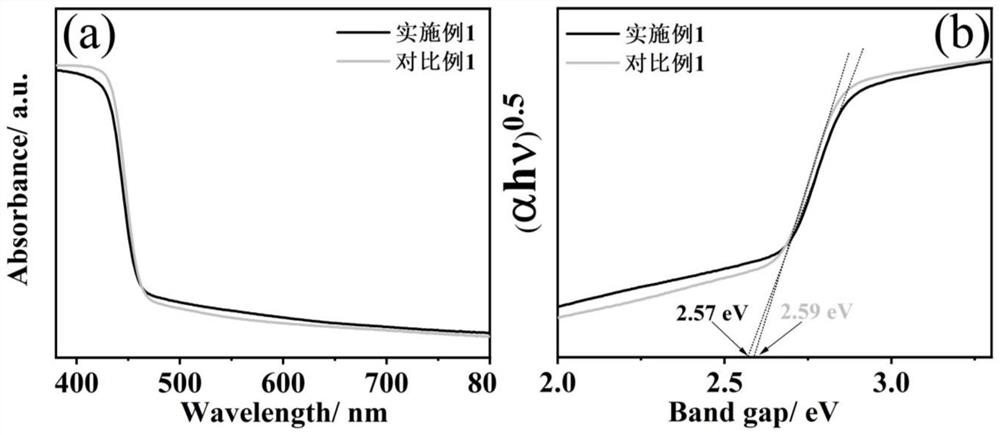
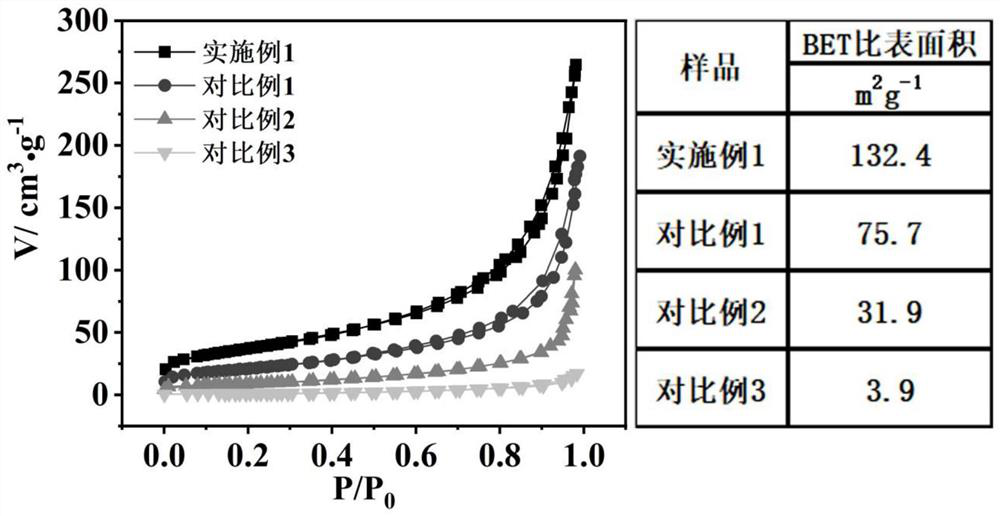
Sulfur-doped crystal carbon nitride for photocatalytic decomposition of water to produce hydrogen and preparation method and application thereof
A technology of sulfur doping and carbon nitride, which is applied in the field of photocatalytic materials, can solve the problems of small specific surface area of crystalline carbon nitride, wide band gap, and limitation of photocatalytic hydrogen production activity, so as to achieve increased sulfur content and narrow band gap , The effect of reducing the duration of molten salt calcination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Weigh 2g of thiocyanic acid and disperse it into 80ml of deionized water, stir at room temperature for 30min, then transfer the dispersion to a 100ml hydrothermal kettle, seal and react at 120°C for 4h, and cool to room temperature after the reaction; Suction filter the product, and freeze-dry the resulting filter cake at -40°C to obtain a precursor;
[0041] (2) Place the precursor in a tube furnace, calcinate at 550°C for 4 hours in a flowing argon atmosphere, and then naturally cool to room temperature to obtain sulfur-doped carbon nitride; the volume space velocity of argon is 2 minutes –1 ;
[0042] (3) The obtained sulfur-doped carbon nitride and molten salt were mixed uniformly by grinding, the mixture was placed in a tube furnace, and calcined at 550°C for 40min under a flowing argon atmosphere; after that, the sample was quickly taken out of the furnace and Rapidly cool to room temperature under the protection of inert gas, wash away excess molten salt wit...
Embodiment 2
[0044] (1) Weigh 1g of thiocyanic acid and 1g of melamine and disperse it into 80ml of deionized water, stir at room temperature for 40min, then transfer the dispersion to a 100ml hydrothermal kettle, seal it and react at 140°C for 2h, cool down after the reaction to room temperature; then the product was filtered with suction, and the resulting filter cake was freeze-dried at -40°C to obtain a precursor;
[0045] (2) Place the precursor in a tube furnace, calcinate at 550°C for 4 hours in a flowing argon atmosphere, and then naturally cool to room temperature to obtain sulfur-doped carbon nitride; the volume space velocity of argon is 2 minutes –1 ;
[0046] (3) The obtained sulfur-doped carbon nitride and molten salt were mixed uniformly by grinding, the mixture was placed in a tube furnace, and calcined at 550°C for 40min under a flowing argon atmosphere; after that, the sample was quickly taken out of the furnace and Rapidly cool to room temperature under the protection o...
Embodiment 3
[0048] (1) Weigh 2g of thiocyanuric acid and disperse it into 80ml of deionized water, stir at room temperature for 30min, then transfer the dispersion to a 100ml hydrothermal kettle, seal and react at 100°C for 3h, and cool to room temperature after the reaction; Suction filter the product, and freeze-dry the resulting filter cake at -40°C to obtain a precursor;
[0049] (2) Place the precursor in a tube furnace, calcinate at 550°C for 4 hours in a flowing argon atmosphere, and then naturally cool to room temperature to obtain sulfur-doped carbon nitride; the volume space velocity of argon is 2 minutes –1 ;
[0050] (3) The obtained sulfur-doped carbon nitride and molten salt were mixed evenly by grinding, the mixture was placed in a tube furnace, and calcined at 550°C for 90min under a flowing argon atmosphere; after that, the sample was quickly taken out of the furnace and Rapidly cool to room temperature under the protection of inert gas, wash away excess molten salt with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com