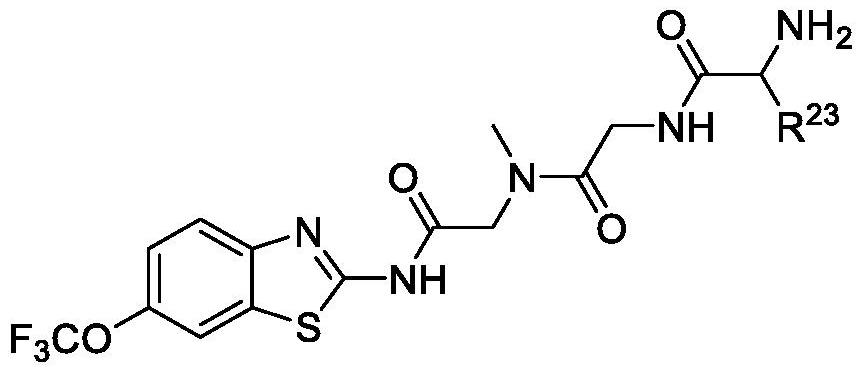
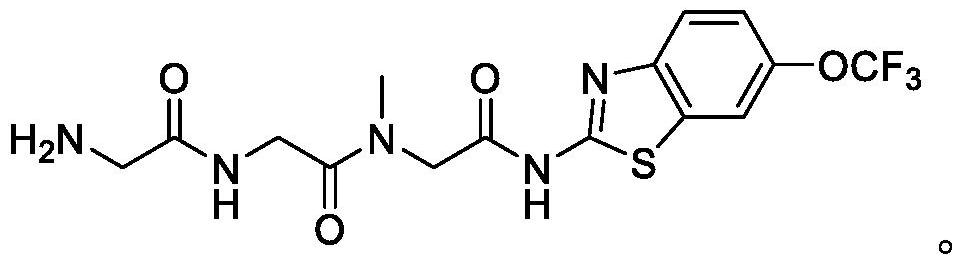
Use of riluzole prodrugs to treat alzheimer's disease
A technology for Alzheimer's disease and riluzole, which is applied in the field of application of riluzole prodrugs in the treatment of Alzheimer's disease, and can solve problems such as clinical benefits of small effect size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Clinical studies were performed using the following parameters. For additional information, see ClinicalTrials.govIdentifier NCT03605667, www.clinicaltrials.gov.
[0086] research description
[0087] Brief overview:
[0088] Preclinical models have shown that riluzole, the active metabolite of BHV-4157, protects against AD-related pathology and cognitive impairment. A titrated dose (to 280 mg) of BHV-4157 or placebo was administered orally once daily. The duration of treatment is 48 weeks. There is also a screening period of up to 42 days and a post-treatment observation period of 4 weeks.
[0089]
[0090] Research design
[0091]
[0092] Groups and Interventions
[0093]
[0094] outcome measures
[0095] Primary Outcome Measures:
[0096] 1. Changes in Alzheimer's Disease Assessment Scale Cognitive Subscale (ADAS-Cog 11) from Baseline to Week 48 between BHV-4157 Treatment Groups and Placebo [Time Frame: Baseline to Week 48] 48 weeks]
[0097] Alz...
Embodiment 2
[0119] Clinical studies were performed using the following parameters.
[0120] Research Summary
[0121]
[0122]
[0123]
[0124] list of abbreviations
[0125] Aβ β-amyloid
[0126] AchEI acetylcholinesterase inhibitor
[0127] AD Alzheimer's disease
[0128] ADAS-Cog Alzheimer's Disease Assessment Scale-Cognition (subscale)
[0129] ADCS-ADL Alzheimer's Disease Collaborative Study - Activity of Daily Living Record Form
[0130] ADME absorption, distribution, metabolism, excretion
[0131] AICD Automatic Implantable Cardioverter Defibrillator
[0132] AUC area under the curve
[0133] AE adverse events
[0134] AIDS acquired immunodeficiency syndrome
[0135] ALS amyotrophic lateral sclerosis
[0136] ALT alanine aminotransferase
[0137] ApoE apolipoprotein E
[0138] AST aspartate aminotransferase
[0139] BDNF brain-derived neurotrophic factor
[0140] BID twice a day
[0141] BUN blood urea nitrogen
[0142] CDR-SOB Clinical Dementia Scale - To...
Embodiment 3
[0344] Capsules containing 140 mg of troluzole for the studies described in Examples 1 and 2 were prepared in the following proportions.
[0345] Composition of 140mg troluzole capsules
[0346]
[0347] 1 Supplied as a 75:25 mixture of microcrystalline cellulose and dicalcium phosphate anhydrous.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com