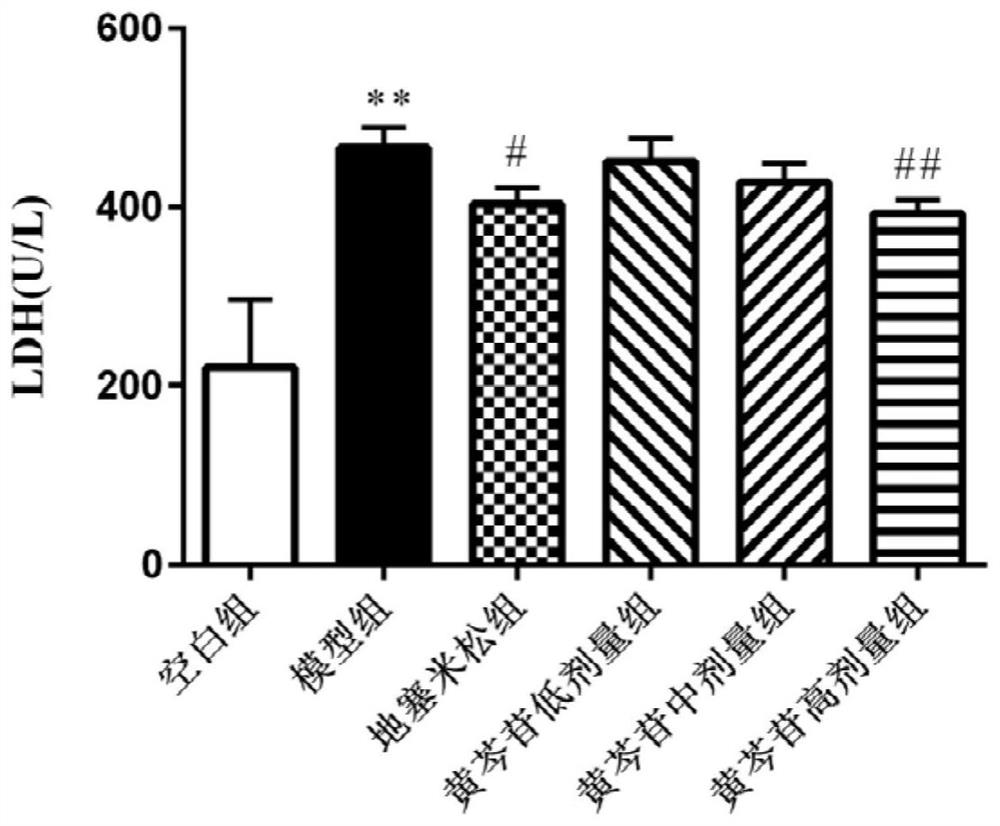
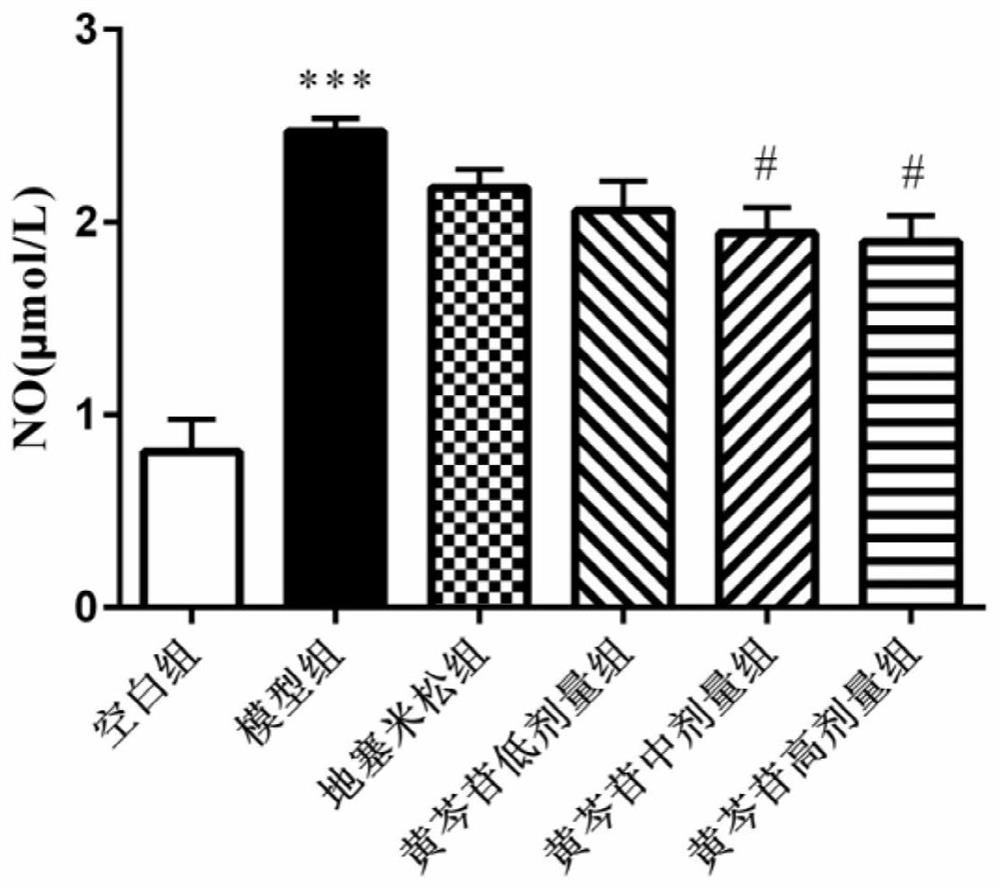
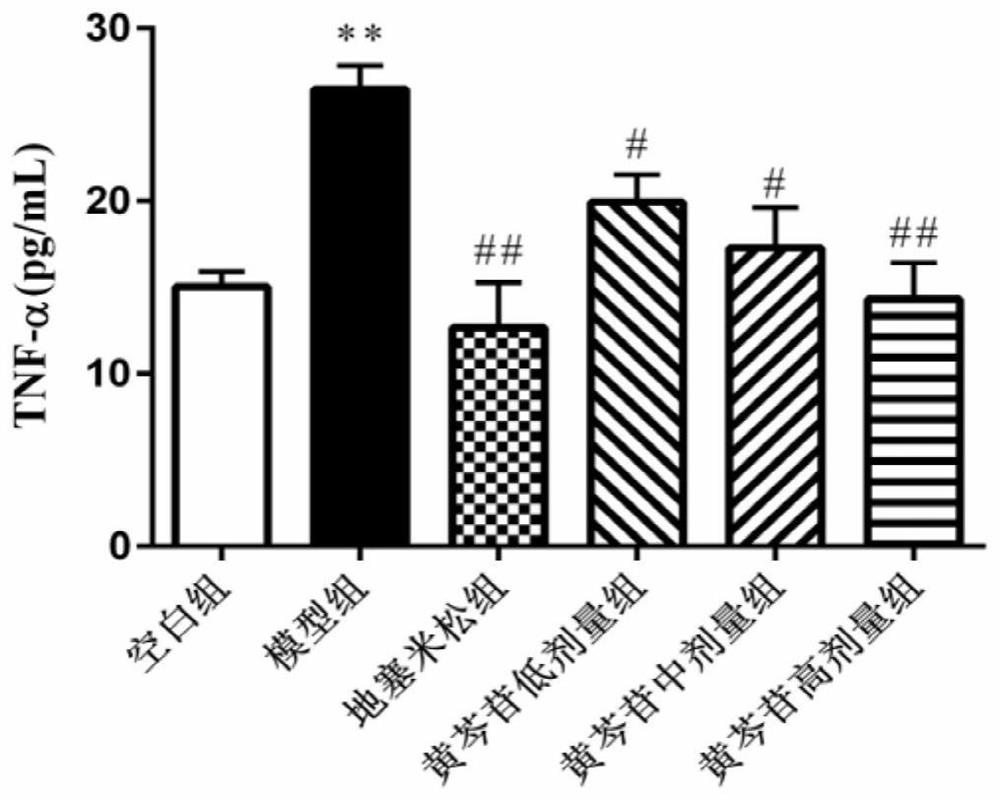
Application of baicalin in preparation of medicine for preventing and/or treating asymptomatic hyperuricemia and/or uric acid nephropathy
A technology for uric acid nephropathy and hyperuricemia, applied in the field of biomedicine, can solve the problems of high toxicity and side effects of uric acid nephropathy drugs, poor tolerance of patients, and clinical use restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0020] The following examples adopt the methods of animal and cell experiments for research.
[0021] 1. Study on the effect of baicalin on uric acid nephropathy in mice
[0022] 1.1 Materials
[0023] 1.1.1 Experimental animals
[0024] Male Kunming mice of SPF grade, weighing 20-22 g, were provided by Spifford (Beijing) Biotechnology Co., Ltd., animal certificate: SCXK (Beijing) 20119-0010.
[0025] 1.1.2 Drugs and reagents
[0026] Baicalin (purity>90%), batch number: C031B150902, purchased from Sichuan Xieli Pharmaceutical Co., Ltd.; allopurinol (purity 98%), batch number: Y27O8C46913, purchased from Shanghai Yuanye Biotechnology Co., Ltd.; oxonic acid potassium salt ( Purity>98%), batch number: P137112, purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.; uric acid assay kit (uricase colorimetric method), batch number: 20191129; urea nitrogen kit (urease method), batch number: 20191211; Creatinine kit (creatinine oxidase method), batch number: 20191218; C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com