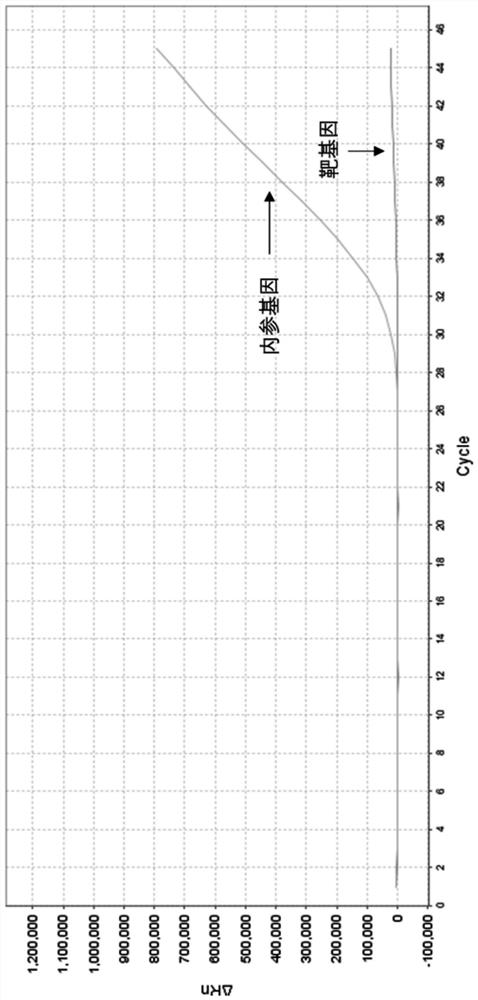
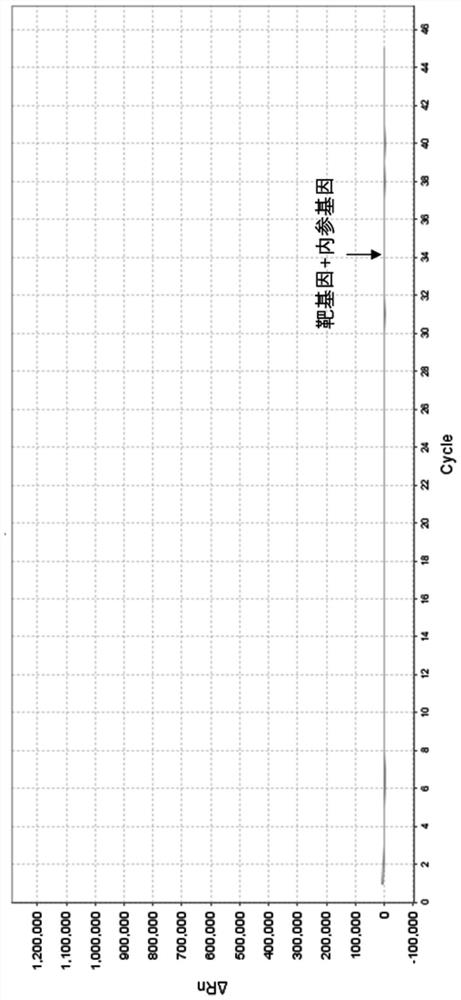
Relative quantification method and kit for detecting copy number of human DMD gene by multiple real-time fluorescence PCR
A gene copy number, real-time fluorescence technology, applied in biochemical equipment and methods, microbial determination/inspection, etc., can solve the problems of mutation hot spots, disappearance, inoperability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] The multiple real-time fluorescent PCR method of the present embodiment detects the test kit (100 reactions / box) of human DMD gene copy number and comprises components as shown in table 2:
[0102] Table 2. Kit components
[0103]
[0104] Exon reaction solution one is the relative quantitative detection primer probe for the 4th, 17th, 45th exon copy number of DMD gene:
[0105] D4F:5'-AAGGCACTGCGGGTTTTG-3',
[0106] D4R: 5'-GTCACAGCATCCAGACCTTGTC-3',
[0107] D4P:5'-FAM-AGAACAATAATGTAAGTAGTACCC-3'-MGB-NFQ,
[0108] D17F: 5'-TGACCTCTGTTTCAATACTTCTCCACA-3',
[0109] D17R: 5'-GTCACCGTAGTTACTGTTTCCATTACA-3',
[0110] D17P:5'-ABY-ACCACCACTCAGCCATCACTAACACAGACA-3'-QSY,
[0111] D45 F: 5'-TCTTCCCCAGTTGCATTCAAT-3',
[0112] D45 R: 5'-CAGGAACTCCAGGATGGCATT-3',
[0113] D45P:5'-VIC-TTCTGACAACAGTTTGCCGCTGCC-3'-QSY,
[0114] CFTR1 F:5'-TTGTGCCTGTTGCAGCTTCT-3',
[0115] CFTR1 R: 5'-TGGAGTTACAGAAAGGCCTCATG-3',
[0116] CFTR1P:5'-Cy5-CGAATGGCACCACCTTCTCGGTGT-3'-QSY,
[...
Embodiment 2
[0155] Adopt the kit described in embodiment 1 to utilize human peripheral blood free DNA or gDNA to carry out quantitative molecular detection to human Duchenne / Becke muscular dystrophy pathogenic gene DMD copy number variation:
[0156] (1) Nucleic acid extraction:
[0157] 1 child with clinical diagnosis of DMD gave birth to 2 female carriers of children with clinical diagnosis of DMD, and also included whole blood samples from 3 normal females and 2 normal males. Use Tianlong Automatic Nucleic Acid Extractor (NP968-3S) and matching Tianlong Whole Blood Genomic DNA Extraction Kit to extract whole blood samples collected in EDTA anticoagulant tubes, and use a micro-ultraviolet spectrophotometer to determine the nucleic acid purity after extraction and concentration, its OD 260 / 280 Between 1.6-2.0; dilute the genomic DNA concentration with sterilized double distilled water to 20ng / μL for later use.
[0158] (2) Dilution of reference substance:
[0159] Dissolve the positiv...
Embodiment 3
[0195] Reagent Specificity Validation: Cross-reactivity to Common Clinical Pathogens.
[0196] (1) Experimental sample
[0197] Take 4 specific samples to verify the specificity of the reagent, hepatitis B virus (10 7 copies / ml), hepatitis C virus (10 5 copies / ml), human cytomegalovirus (10 4 copies / ml), Enterovirus 71 (10 4 copies / ml).
[0198] (2) Experimental process
[0199]Use the first reaction solution to the third reaction solution to test the above four specific samples respectively, analyze the test results, and verify the specificity of the reagents.
[0200] (3) Experimental results
[0201] The 4 specific samples tested by the 3 kinds of reaction solutions were all Undetermined, indicating that the specificity was good and there was no cross-reaction. The specific results are shown in Table 7.
[0202] Table 7. Results of cross-reaction between the three reaction solutions and common clinical pathogens (UD=Undetermined)
[0203]
[0204]
[0205] In ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com