A method for separating sodium and lithium from partially ionized sodium-lithium-containing brine
A brine and salt lake brine technology, applied in chemical instruments and methods, lithium halide, alkali metal sulfite/sulfite, etc., can solve the problem that LiOH crystals contain a lot of NaOH impurities, the separation of lithium and sodium is unclear, and the washing is difficult. and other problems, to achieve the effect of overcoming the conversion obstacles, reducing the amount of evaporated water, and ensuring the separation of sodium and lithium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
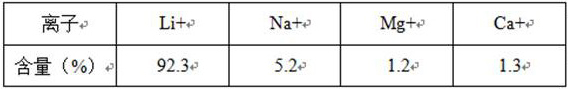
[0030] S1: The salt lake brine containing NaCl and LiCl system (n Li+ / n Na+ =8.3) Perform partial electrolysis to obtain LiCl and NaOH solution system, ensuring that 0.98≤nOH in the system - / nNa + ≤1.1;
[0031] S2: Add H to LiOH and LiCl solution system 2 SO 4 , join H 2 SO 4 with Na + The molar ratio is 1:4 to get LiCl and Na 2 SO 4 solution system;
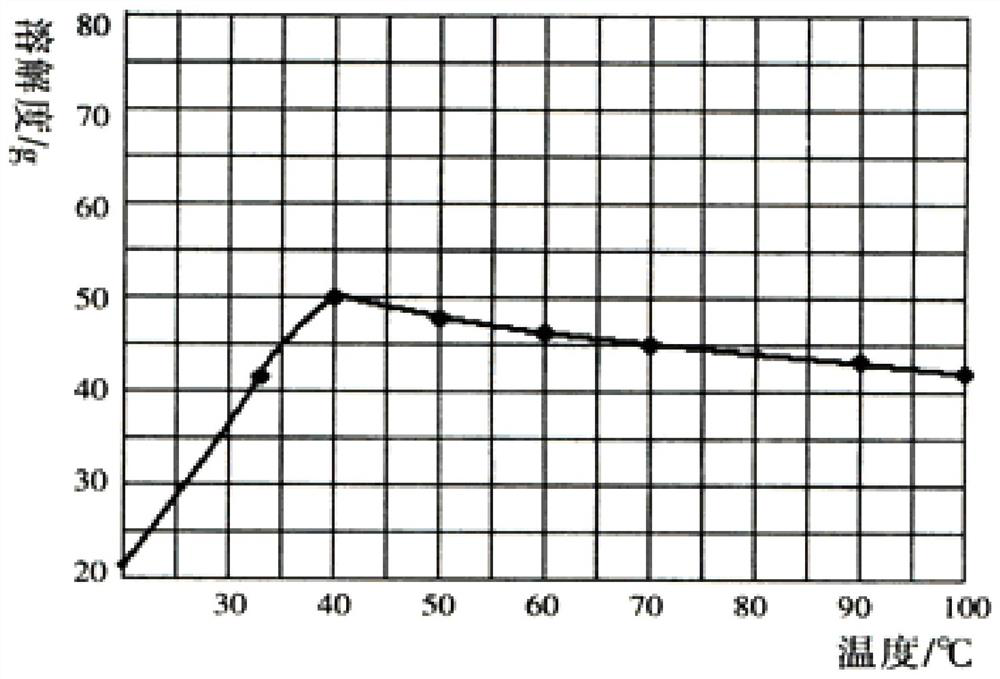
[0032] S3: LiCl and Na 2 SO 4 The solution is evaporated, the evaporation temperature is 40 ° C, so that Na 2 SO 4 In a saturated state, the solution to be frozen is obtained;
[0033] S4: adding a freezing aid to the solution to be frozen; the freezing aid is ethanol;
[0034] S5: The liquid to be frozen with the freezing auxiliary agent is subjected to freezing and crystallization treatment, the freezing temperature is -20°C, and centrifuged to obtain LiCl lithium liquid and Na 2 SO 4 10H 2 O crystals.
Embodiment 2
[0036] S1: The salt lake brine containing NaCl and LiCl system (n Li+ / n Na+ =6.5) Perform partial electrolysis to obtain LiCl and NaOH solution system, ensuring that 0.98≤nOH in the system - / nNa + ≤1.1;
[0037] S2: Add H to LiCl and NaOH solution system 2 SO 4 , join H 2 SO 4 with Na + The molar ratio is 1:4 to get LiCl and Na 2 SO 4 solution system;
[0038] S3: LiCl and Na 2 SO 4 The solution is evaporated, and the evaporation temperature is 80°C, so that Na 2 SO 4 In a saturated state, the solution to be frozen is obtained;
[0039] S4: adding a freezing aid to the solution to be frozen; the freezing aid is isopropyl;
[0040] S5: The liquid to be frozen with the freezing auxiliary agent is subjected to freezing and crystallization treatment, the freezing temperature is -10°C, and centrifuged to obtain LiCl lithium liquid and Na 2 SO 4 10H 2 O crystals.
Embodiment 3
[0042] S1: The salt lake brine containing NaCl and LiCl system (n Li+ / n Na+ =8.8) Perform partial electrolysis to obtain LiCl and NaOH solution system to ensure that 0.98≤nOH in the system - / nNa + ≤1.1;
[0043] S2: Add H to LiCl and NaOH solution system 2 SO 4 , join H 2 SO 4 with Na + The molar ratio is 1:4 to get LiCl and Na 2 SO 4 solution system;
[0044] S3: LiCl and Na 2 SO 4 The solution is evaporated, the evaporation temperature is 100 ° C, so that Na 2 SO 4 In a saturated state, the solution to be frozen is obtained;
[0045] S4: adding a freezing aid to the solution to be frozen; the freezing aid is ethylamine;
[0046] S5: The liquid to be frozen with the freezing auxiliary agent is subjected to freezing and crystallization treatment, the freezing temperature is 5°C, and centrifuged to obtain LiCl lithium liquid and Na 2 SO 4 10H 2 O crystals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com