Improved process for preparation of intermediates
A technology of system and solvent, which is applied in the field of preparation of 2--2-[methyl]oxirane, can solve problems such as insufficient yield and unsuitable reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
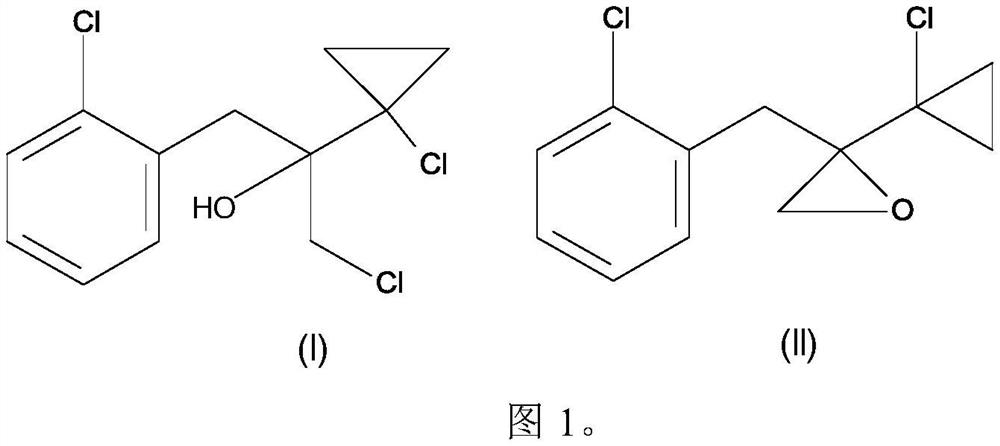
[0107] Example 1: 2-(2-chlorobenzyl)-2-(1-chlorocyclopropyl)oxirane and 1-chloro-2-(1-chlorocyclopropyl)-3- Preparation of (2-chlorophenyl)propan-2-ol (according to the invention)
[0108] 2- A mixture of chlorobenzyl chloride (163.0 g, 1.0 mol) in toluene (230 mL) and 2-methyltetrahydrofuran (110 mL). The reaction mixture was cooled to 0°C to 10°C, and a solution of 1-chloro-1-chloroacetylcyclopropane (153 g, 0.95 mol) in 2-methyltetrahydrofuran (40 ml) and toluene (120 ml) was added dropwise thereto , and the reaction was stirred for 2 hours. The mixture of toluene and 2-methyltetrahydrofuran was concentrated under reduced pressure, and the reaction mass was cooled to 10°C. Water (350ml) was added to the reaction mass, followed by hydrochloric acid (30%, 170g), and stirred at room temperature for 3 hours. The organic layer was separated, dried and concentrated under reduced pressure to obtain 247 g of 2-(2-chlorobenzyl)-2-(1-chlorocyclopropyl)oxirane and 1-chloro-2-(1...
Embodiment 2
[0109] Example 2: 2-(2-chlorobenzyl)-2-(1-chlorocyclopropyl)oxirane and 1-chloro-2-(1-chlorocyclopropyl)-3- Preparation of (2-chlorophenyl)propan-2-ol (comparative example according to US5099040)
[0110] A mixture of magnesium flakes (17.0 g, 0.708 mol) and iodine (0.5 g) was treated with toluene (80 ml), tetrahydrofuran (20 ml) and 2-chlorobenzyl chloride (1 g, 0.006 mol) at 20°C. At 50°C to 55°C, a mixture of 2-chlorobenzyl chloride (97.0 g, 0.60 mol) in toluene (338 ml) and tetrahydrofuran (42 ml) was added dropwise thereto within 5 hours. After the addition was complete, the mixture was reacted at 50°C to 55°C for 30 minutes. The reaction mixture was cooled to 20°C, and unreacted magnesium was decanted. To the decanted reaction mixture was added 1-chloro-1-chloroacetylcyclopropane (89 g, 0.58 mol) dropwise over 45 minutes at 20°C to 30°C, and the reaction was stirred for a further 30 minutes. The reaction mixture was poured into a solution of concentrated sulfuric aci...
Embodiment 12
[0116] Embodiment 12: the preparation of prothioconazole :
[0117] Step a): 2-(2-chlorobenzyl)-2-(1-chlorocyclopropyl)oxirane and 1-chloro-2-(1-chlorocyclopropyl)-3-(2- Preparation of Chlorophenyl)propan-2-ol
[0118] Follow the method of Example 1 to prepare 2-(2-chlorobenzyl)-2-(1-chlorocyclopropyl)oxirane and 1-chloro-2-(1-chlorocyclopropyl)-3- (2-Chlorophenyl)propan-2-ol mixtures.
[0119] Step b): Preparation of 2-(1-chlorocyclopropyl)-1-(2-chlorophenyl)-3-(1,2,4-triazol-1-yl)propan-2-ol prepare :
[0120] A mixture of 1,2,4-triazole (431 g), potassium carbonate (861 g) in dimethylformamide (DMF) (704 g) was heated to 80°C to 85°C for 1.0 hour. To this mixture were added dropwise 1-chloro-2-(1-chlorocyclopropyl)-3-(2-chlorophenyl)propan-2-ol and 2-(2-chlorobenzyl)-2-(1- Chlorocyclopropyl)oxirane (577 g) in DMF (450 g) and the reaction mixture was heated to 80°C to 85°C for 3 hours. The mixture was then cooled to room temperature and filtered to give a resid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com