Preparation method of 3beta-acetoxyandrostane-5-ene-17-one
A technology of acetoxyandrostene and androstane, which is applied in the field of organic compound preparation, can solve the problems of many by-products and low total yield, and achieve the effects of convenient operation, cost reduction, and enhancement of market competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
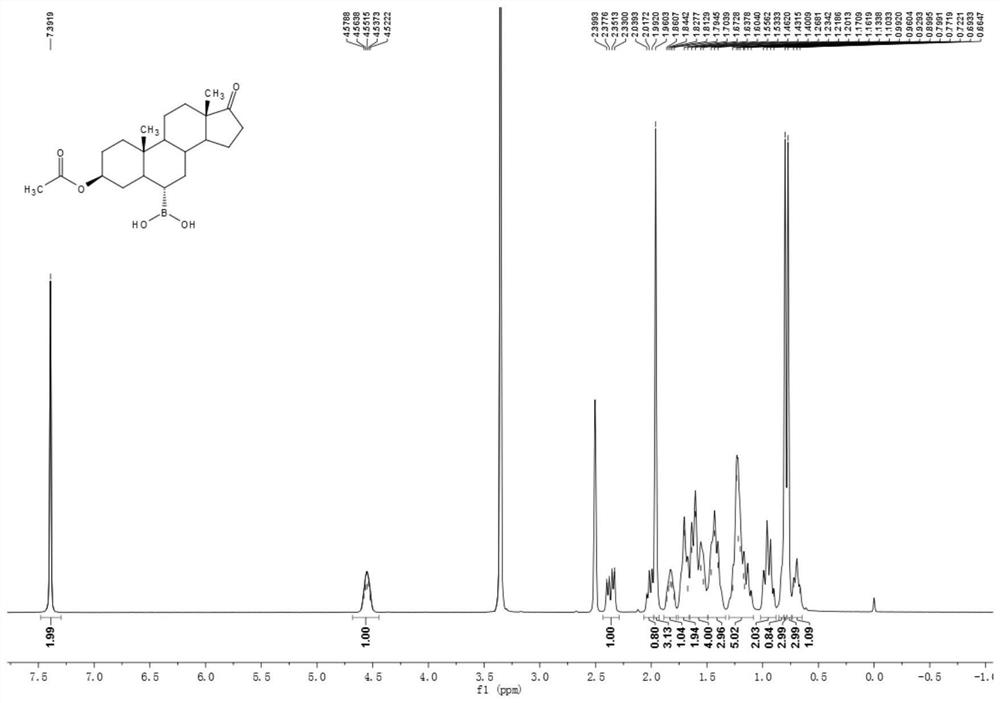
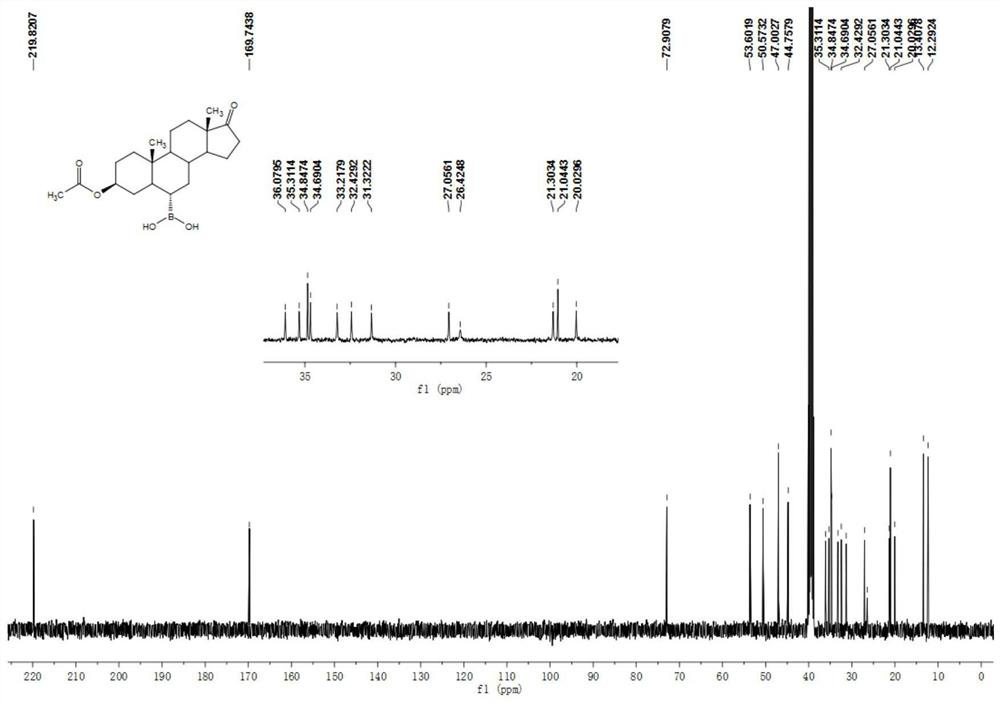
[0050] Example 1 Preparation of 3β-acetoxyandrost-17-one-6α-boronic acid
[0051] Add 10g of the compound androstane-3β-hydroxy-17-one-6α-boronic acid, 30ml of acetic anhydride and 10ml of pyridine into a 100ml three-necked flask, heat up to 80°C for 1 hour under stirring, and the reaction of the raw materials is complete. Cool down to room temperature, add 70ml of water, solid precipitates, stir for 15 minutes, filter with suction, wash with water until neutral, and dry to obtain 11g of white solid compound 3β-acetoxyandrost-17-one-6α-boronic acid. 1 H NMR (400MHz, DMSO-d6 ):δ7.39(s,2H),4.58-4.52(m,1H),2.35(dd,J=19.2,8.7Hz,1H),2.04-1.99(m,1H),1.96(s,3H), 1.86-1.79(m,1H),1.71-1.67(m,2H),1.64-1.53(m,4H),1.46-1.40(m,3H),1.27-1.10(m,5H),0.99-0.90(m ,2H),0.87-0.81(m,1H),0.80(s,3H),0.77(s,3H),0.72-0.66(m,1H). 13 C NMR (100MHz, DMSO-d 6 ): δ 219.8, 169.7, 72.9, 53.650.6, 47.0, 44.8, 36.1, 35.3, 34.9, 34.7, 33.2, 32.4, 31.3, 27.1, 26.4, 21.3, 21.0, 20.0, 13.4, 12.3.
Embodiment 2
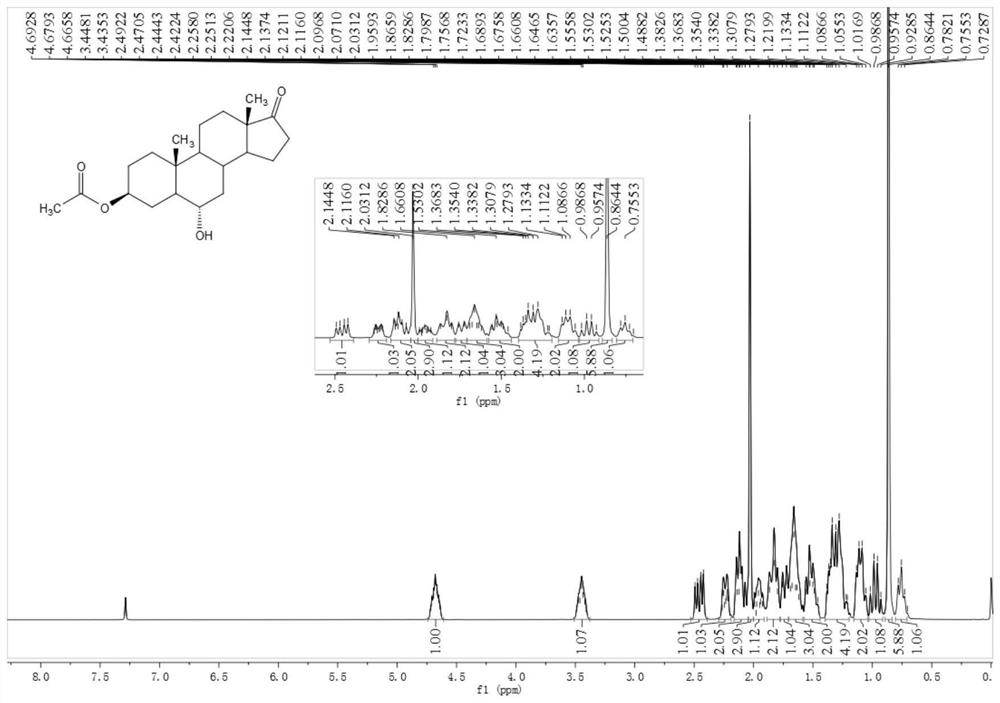
[0052] Example 2 Preparation of 3β-acetoxyandrost-6α-hydroxyl-17-one
[0053] Add 11g of compound 3β-acetoxyandrost-17-one-6α-boronic acid and 25ml of methanol into a 100ml three-necked flask, stir to dissolve, cool down to 10°C, add 4g of 30% hydrogen peroxide, stir for 30 minutes, heat up to 20°C for reaction After 2 hours, the raw material reacted completely and was extracted with 10% sodium bisulfite. Recover methanol under reduced pressure, evaporate to dryness, add 40ml of water and 50ml of dichloromethane, stir and wash for 5 minutes to separate layers, wash with water (10ml×3) to pH = 7, add 10g of anhydrous sodium sulfate to dry, suction filter, and recover dichloromethane under reduced pressure methane to obtain 9.7 g of oily 3β-acetoxyandrost-6α-hydroxyl-17-one. 1 H NMR (400MHz, CDCl 3 ):δ4.71-4.65(m,1H),3.51-3.38(m,1H),2.46(dd,J=19.2,8.7Hz,1H),2.26-2.21(m,1H),2.14-2.10(m ,2H),2.03(s,3H),1.99-1.92(m,1H),1.87-1.80(m,2H),1.76-1.72(m,1H),1.69-1.61(m,3H),1.56-1.46 (...
Embodiment 3
[0054] Example 3 Preparation of 3β-acetoxyandrost-17-one-6α-ol p-toluenesulfonate
[0055] Add 9.7 g of the above-mentioned oily 3β-acetoxyandrost-6α-hydroxy-17-one and 30 ml of pyridine into a 100 ml three-necked flask, and stir to dissolve. Cool down to 0°C and add 8g of p-toluenesulfonyl chloride, stir and react at room temperature for 12 hours, and the reaction is complete. Add 100ml of water, stir, solid precipitates out, filter with suction, wash with water, and dry to obtain 13.0g of 3β-acetoxyandrost-17-one-6α-ol p-toluenesulfonate as a white solid. 1 H NMR (400MHz, CDCl 3 ): δ7.77(d, J=7.8Hz, 2H), 7.33(d, J=7.8Hz, 2H), 4.58-4.51(m, 1H), 4.45-4.39(m, 1H), 2.47-2.40( m,4H),2.19-2.16(m,1H),2.10-2.03(m,1H),1.99(s,3H),1.85-1.78(m,4H),1.73-1.60(m,2H),1.52- 1.15(m,8H),1.08-0.95(m,2H),0.84(s,6H),0.74-0.69(m,1H). 13 C NMR (100MHz, CDCl 3 ):δ220.2,170.3,144.6,134.4,129.7(2C),127.7(2C),81.2,72.4,53.3,50.9,48.7,47.7,37.7,37.2,36.7,35.7,34.0,31.2,28.2,26.8,21.7, 21.6, 21.3, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com