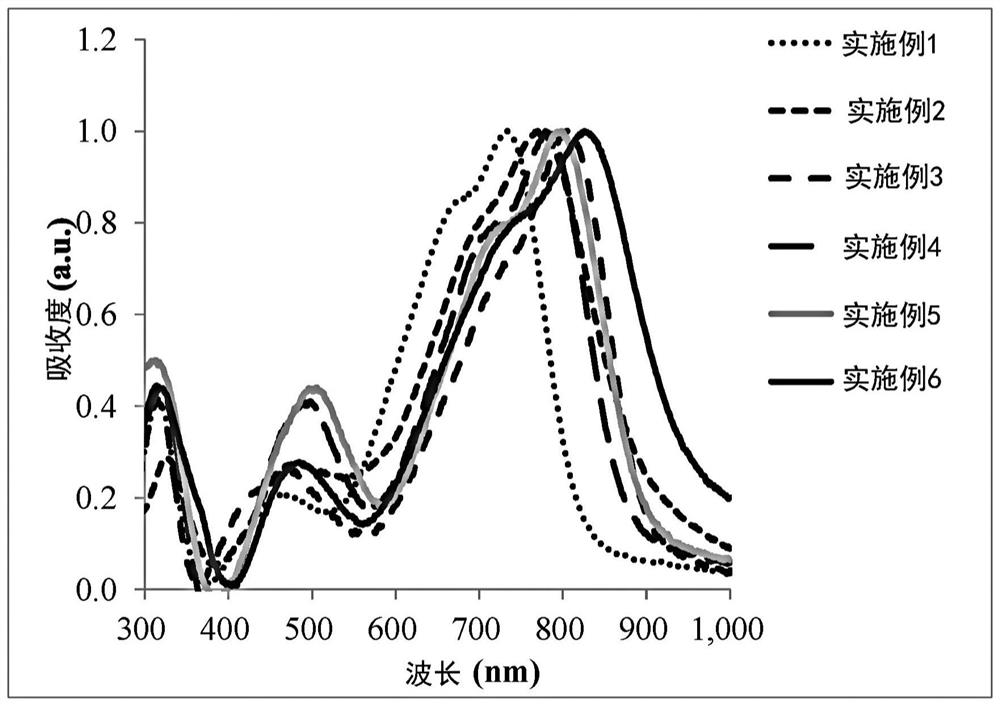
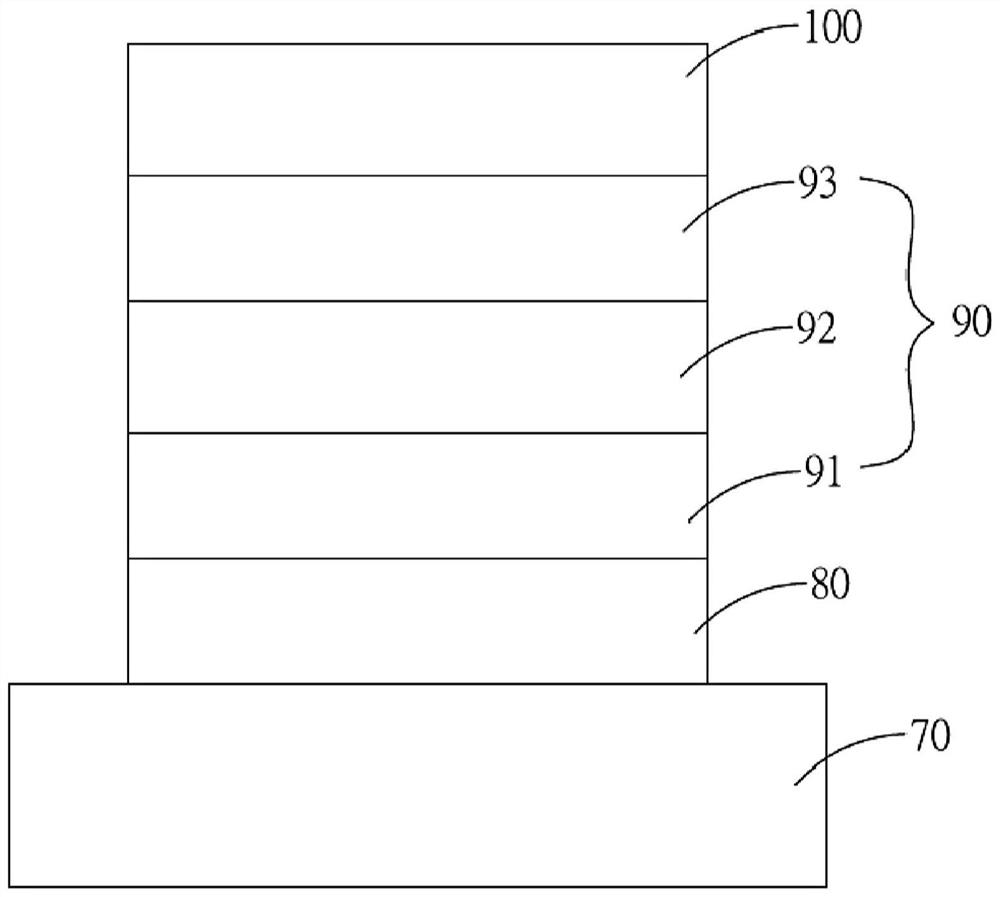
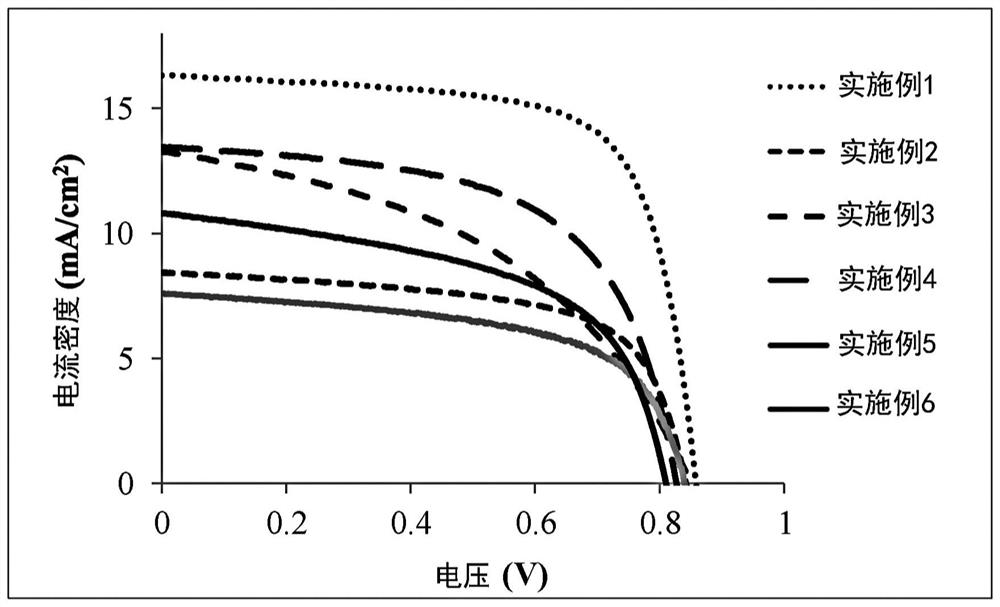
Non-fullerene electron acceptor material and organic photovoltaic cell
A technology of electron acceptor materials and organic photovoltaic cells, which is applied in photovoltaic power generation, organic chemistry, electric solid devices, etc., can solve the problems of raw material toxicity and production method pollution, low energy conversion efficiency of organic electron acceptor materials, and unsatisfactory , to achieve the effect of improving energy conversion efficiency and excellent photoelectric conversion characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Preparation of non-fullerene electron acceptor materials
[0060] The preparation process of the non-fullerene electron acceptor material of Example 1 is shown in the following reaction formula I.
[0061] [Reaction Formula I]
[0062]
[0063] Compound 2
[0064]
[0065] The preparation method of compound 2:
[0066] 3-Chlorothiophene (compound 1) (10 g, 84.3 mmol) was dissolved in 300 mL of tetrahydrofuran (THF). At -40°C, n-butyllithium (n-BuLi) (30.6 mL, 76.66 mmol) was slowly added dropwise and stirred for 1 hour. Trimethyltin chloride (Me 3 SnCl) (15.27g, 76.66mmol), returned to room temperature (rt) and stirred for 15 minutes and the reaction was completed. Next, it was extracted three times with n-heptane and water. The organic layer was extracted with saturated brine, dehydrated with anhydrous magnesium sulfate, and dried with a cyclone concentrator to obtain compound 2 (18 g, yield: 76%) as a yellow liquid.
[0067] Compound 4
[0068]
[006...
Embodiment 2
[0104] Non-Fullerene Electron Acceptor Materials
[0105] The preparation process of the non-fullerene electron acceptor material of Example 2 is shown in the following reaction formula II.
[0106] [Reaction II]
[0107]
[0108] Compound 14
[0109]
[0110] The preparation method of compound 14:
[0111] Compound 8 (5 g, 13.9 mmol) was added to 100 mL of methanol (MeOH). Then 6N aqueous sodium hydroxide solution (23.3 mL, 139.5 mmol) was added and heated to reflux for 16 hours. After cooling, methanol was removed using vortex concentration. Under ice bath, acidify with concentrated hydrochloric acid to pH 1-2, white solid precipitates out and is filtered. White solid compound 14 (4.27 g, yield: 93%) was obtained after vacuum drying.
[0112] Compound 15
[0113]
[0114] The preparation method of compound 15:
[0115] 2,5-bisacid bisthiophene (compound 14) (4 g, 12.1 mmol) was mixed with 100 mL of dichloromethane (DCM). Under ice bath, add oxalyl chloride ...
Embodiment 3
[0142] Non-Fullerene Electron Acceptor Materials
[0143] The preparation process of the non-fullerene electron acceptor material of Example 3 is shown in the following reaction formula III.
[0144] [Reaction III]
[0145]
[0146] Compound 22
[0147]
[0148] The preparation method of compound 22:
[0149]5,6-Dichloro1,3-indandione (Compound 21) (2 g, 9.3 mmol) and malononitrile (1.23 g, 18.6 mmol) were dissolved in 40 mL of ethanol (EtOH). Sodium acetate (NaOAc) (1.14 g, 14 mmol) was added and stirred at room temperature for 16 hours. Next, an aqueous hydrochloric acid solution was added for acidification, and the resulting solid was filtered. The solid was purified by silica gel column chromatography (dichloromethane as the eluent), and dried in vacuo to obtain compound 22 as a brown solid (1.94 g, yield: 78%).
[0150] Example 3
[0151]
[0152] The preparation method of embodiment 3:
[0153] Compound 13 (500 mg, 0.4 mmol) and compound 22 (562.3 mg, 2.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com