Alginate microcapsules for cell encapsulation and, manufacturing method therefor
A technology of alginate and microcapsules, which is applied in the field of surface modified alginate microcapsules, which can solve problems such as melting, weak binding force, and weak physical properties, so as to minimize immune reactions, improve protection, and improve cell survival rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0082] And, the present invention provides the preparation method of following surface modified microcapsules: comprise: core preparation step (1), prepare calcium alginate microcapsule; Shell preparation step (2), by making calcium alginate microcapsule and epigallocate Theophin gallate dimer reacts to form alginate epigallocatechin gallate dimer cross-linked product on the surface of the above-mentioned calcium alginate microcapsule; and nuclear liquefaction step (3), the calcium alginate The calcium ions are chelated to the epigallocatechin gallate dimer to chelate the calcium ions of calcium alginate, the core is fluid alginate, and the shell is composed of epigallocatechin gallate dimer Cross-linked alginate hydrogel.
[0083] First, calcium alginate microcapsules are prepared.
[0084] Next, the calcium alginate microcapsules and the epigallocatechin gallate dimer were reacted. As a result of the above reaction, the alginate of the calcium alginate hydrogel is cross-li...
Synthetic example 1
[0108] Synthesis example 1. Preparation of epigallocatechin gallate (EGCG) dimer
[0109] 2,2-diethoxyethylamine (2,2-diethoxyethylamine, DA) exists as an aldehyde in an acidic environment. Epigallocatechin was prepared by reacting epigallocatechin gallate (EGCG) with 2,2-diethoxyethylamine under methanesulfonic acid (MSA) to cause aldehyde-mediated polymerization Theophyl gallate dimer.
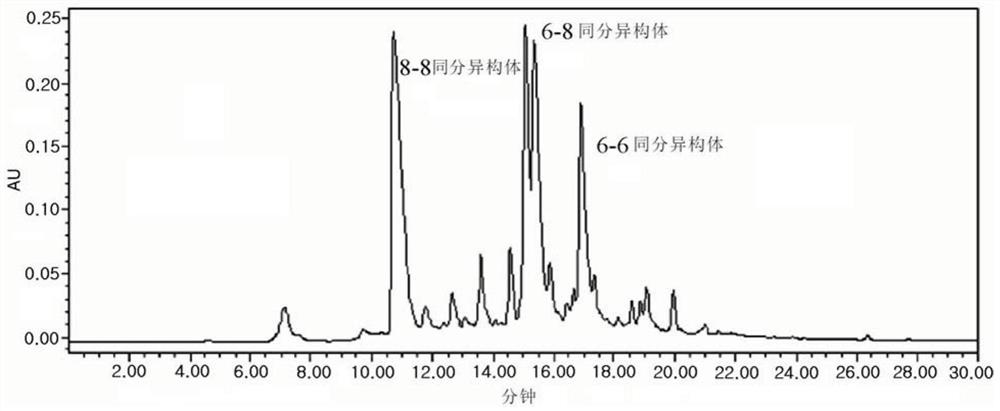
[0110] Specifically, 3.8 ml of tetrahydrofuran and 7 μl of methanesulfonic acid were mixed in a 10 ml vial, and 2.29 g (5 mmol) of epigallocatechin gallate was added under nitrogen flow in a dark room, followed by stirring for 1 to 2 hours. Next, in a cooled 10 ml vial, 145 μl of 2,2-diethoxyethylamine was added to a mixed solvent of 1 ml of tetrahydrofuran and 0.2 ml of methanesulfonic acid and stirred for 20 to 30 minutes to remove aminoacetaldehyde acetaldehyde. The ethoxy group in diethyl and exposes the aldehyde group. Slowly drip the 2,2-diethoxyethylamine solution from which the et...
Synthetic example 2
[0115] Synthesis Example 2. Preparation of Alginate-Epigallocatechin Gallate Conjugate
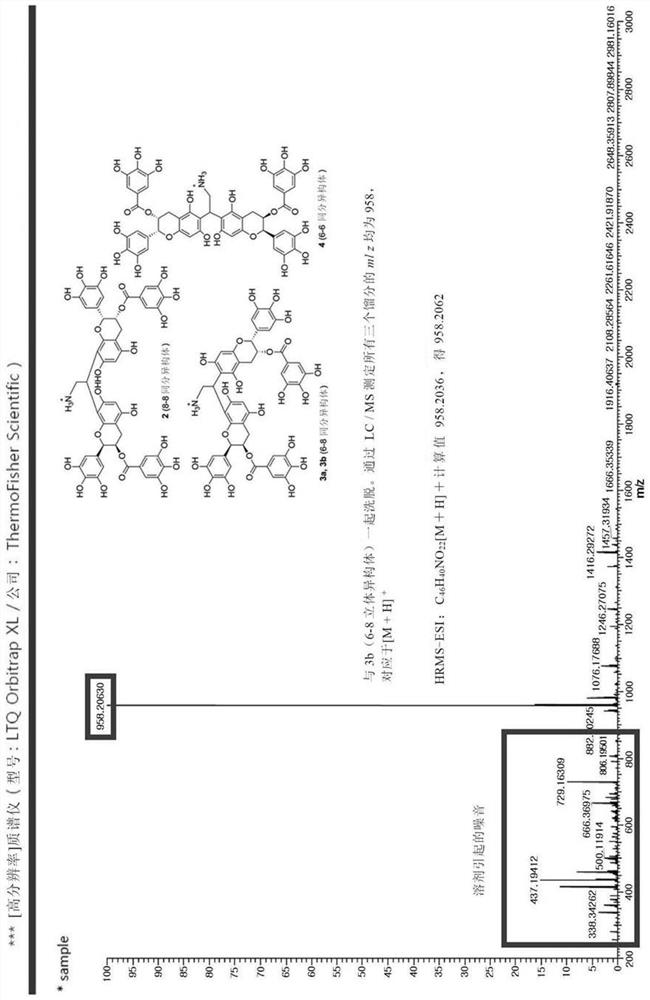
[0116] Using the coupling reaction mediated by carbodiimide, the epigallocatechin gallate dimer was bonded to a polymer having a carboxylic acid group by the method of Reaction Formula 2. In the presence of N-hydroxysuccinimide (NHS), N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide (EDC) under acidic conditions (pH4.7), the algal Alginate reacts overnight with epigallocatechin gallate dimer to form an amide bond between the amine group of epigallocatechin gallate and the carboxylic acid of HA to form an amide bond To prepare alginate-epigallocatechin gallate conjugate.
[0117] Reaction 2
[0118]
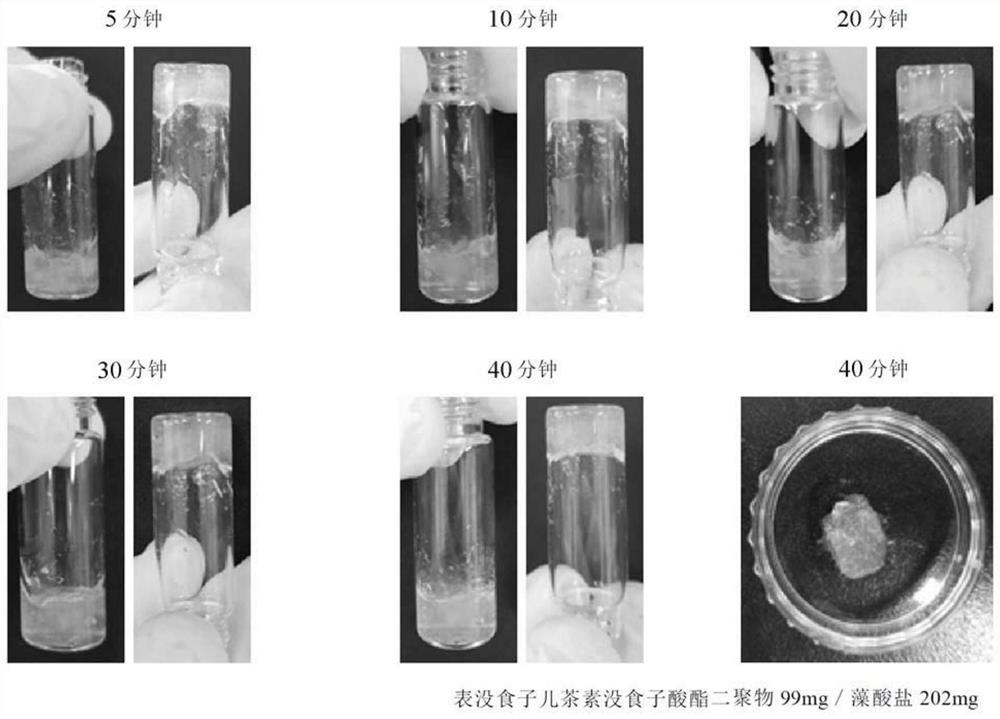
[0119] Specifically, under dark room conditions, in a 3-necked flask, 202 mg of algal salt and stir. After the alginate was completely dissolved, the inside of the flask was replaced with a nitrogen atmosphere and sealed. After dissolving 89 mg of N-hydroxysuccinimide (0.78 mmol) in 3 ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com