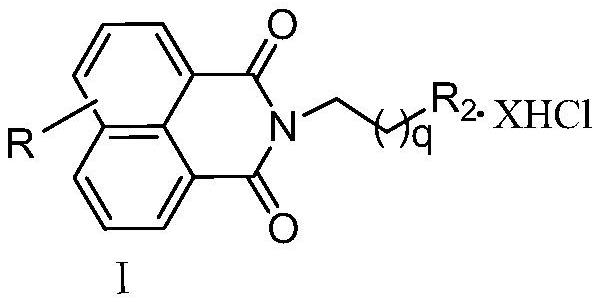
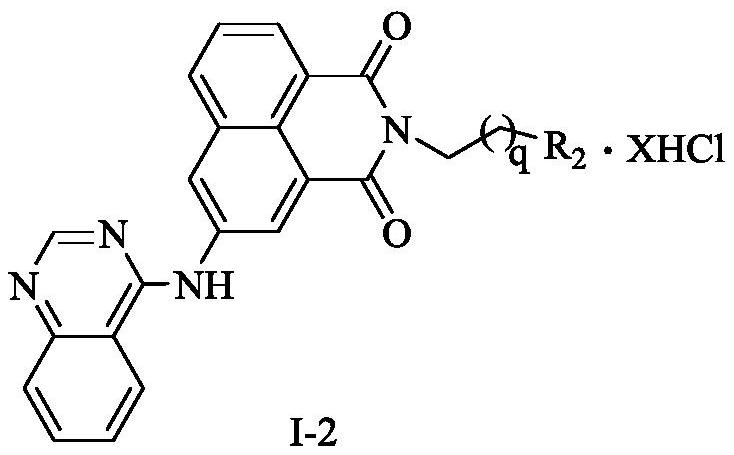
Naphthalimide-polyamine conjugate as well as preparation method and application thereof
A technology of naphthalimide and conjugates, which is applied in the field of naphthalimide-polyamine conjugates and its preparation, and can solve the problem that the side effects of bone marrow suppression are difficult to alleviate or eliminate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
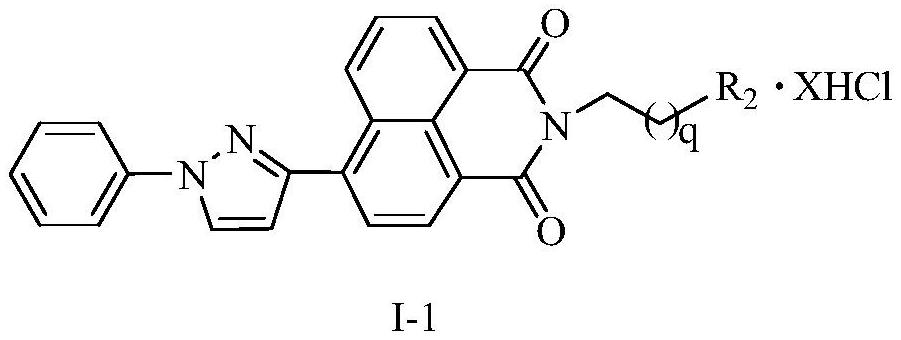
[0045]6{3-(1-Phenyl-1H-pyrazole)}-2-[4-4-(4-aminobutyl)-aminobutyl-aminobutyl]1H-benzisoquinoline-1, Synthesis of 3(2H)-diketone tetrahydrochloride (14a)
[0046]
[0047](1) Take 1.0g (6.5mmol) of compound 8, anhydrous AlCl3 1.29g (9.7mmol) was placed in a 100mL round bottom flask, dry dichloromethane was added as the reaction solvent, after stirring at room temperature for 15 minutes, slowly drop 0.48mL of dichloromethane solution containing 6.8mmol of acetyl chloride under ice bath conditions, dropwise After reaction at room temperature for 30 minutes. After 2h, pour into ice water, extract the organic layer with dichloromethane, dry with anhydrous sodium sulfate, concentrate under reduced pressure, petroleum ether: ethyl acetate = 10:1 column separation and purification to obtain compound 9;
[0048](2) Weigh 1.3g (6.7mmol) of compound 9 obtained in step (1) into a 50mL round-bottomed flask, add 7mL DMF-DMA as the reactant and as the solvent at the same time, reflux for 1h, and evaporate...
Embodiment 2
[0054]6{3-(1-phenyl-1H-pyrazole)}-2-[3-3-(3-aminopropyl)-aminopropyl-aminopropyl]1H-benzisoquinoline-1, Synthesis of 3(2H)-diketone tetrahydrochloride (14b)
[0055]
[0056]Except that 5a is used instead of 5b in step (4), the other synthesis and purification methods are the same as in Example 1. The yield was 52%.
[0057]1H NMR (300MHz, DMSO-d6) δ: 8.46 (dd, J = 9.0, 6.0 Hz, 2H), 8.08 (d, J = 9.0 Hz, 1H), 7.98 (d, J = 3.0 Hz, 1H), 7.77 (dd,J=15.0,7.5Hz,2H),7.25-7.18(m,5H),6.83(s,1H),4.10(t,J=6.0Hz,2H),3.01-2.88(m,10H), 2.08-1.97(m,6H).13C NMR(75MHz,DMSO-d6)δ163.96,163.75,141.04,139.72,139.17,134.96,132.06,131.49,130.37,130.17,130.01,129.56,128.45,128.14,128.08,124.75, 123.04,122.93,111.44,45.25,44.30,44.22,37.71,36.32,24.88,23.89,22.63.ESI-MS m / z:511.47[M-4HCl+1]+.Elemental analysisfor C30H38Cl4N6O2: C, 54.89; H, 5.83; N, 12.80; Found: C, 54.73; H, 6.18; N, 12.73.
Embodiment 3
[0059]6{3-(1-phenyl-1H-pyrazole)}-2-[4-(4-aminobutyl)-aminobutyl]1H-benzisoquinoline-1,3(2H)-di Synthesis of ketone trihydrochloride (14c)
[0060]
[0061]Except that 3d is used instead of 5b in step (4), the other synthesis and purification methods are the same as in Example 1. The yield was 58%.
[0062]1H NMR(300MHz,DMSO-d6)δ:8.49-8.41(m,2H),8.12-8.03(m,1H),7.98(d,J=3.0Hz,1H),7.77(dd,J=13.5,7.5 Hz,2H),7.25-7.17(m,5H),6.84(d,J=3.0Hz,1H),4.05(s,2H),2.95-2.82(m,4H),2.79(d,J=6.0Hz ,2H),1.66(d,J=18.0Hz,8H).13C NMR (75MHz, DMSO-d6) δ163.77,163.58,141.05,139.70,139.16,134.96,132.08,131.53,130.42,130.20,130.00,129.56,128.48,128.06,124.73,122.94,122.83,111.43,46.74,46.26,38.28 ,25.21,24.34,23.59,22.84.ESI-MS m / z:482.43[M-3HCl+1]+.Elemental analysisfor C29H34Cl3N5O2·0.3H2O: C, 58.41; H, 5.85; N, 11.74; Found: C, 58.92; H, 5.52; N, 11.58.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com