Novel derivatization reagent, method for testing sulfur-containing component and application
A technology for derivatizing reagents and testing methods, applied in the field of instrumental analysis, can solve the problems of lack of separation pretreatment and inaccurate test results, etc., and achieve the effect of accurate control of reagent addition and derivation time, and accurate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
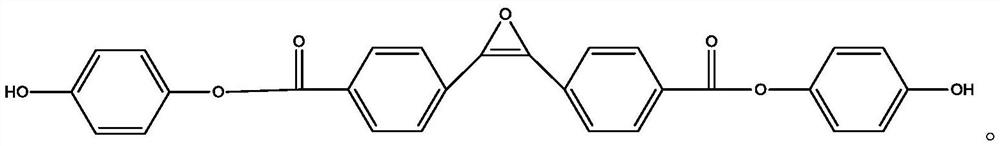
[0067] Synthetic derivatization reagents.
[0068] 4,4-Diphenylethylene dicarboxylic acid (11.3g, 50mmol), hydroquinone (13.2g, 120mmol) and methanesulfonic acid (0.48g, 10mmol%) were sequentially added to the reaction tube. Add 50 mL of acetone to the reaction bottle and stir to dissolve at room temperature, move the reaction bottle to an oil bath at 60°C and heat to reflux for 2 hours, return the reaction system to room temperature, wash with water, remove the solvent by rotary evaporation, wash with n-hexane, and recrystallize to 93 % (21.01 g) yield gave Intermediate K.
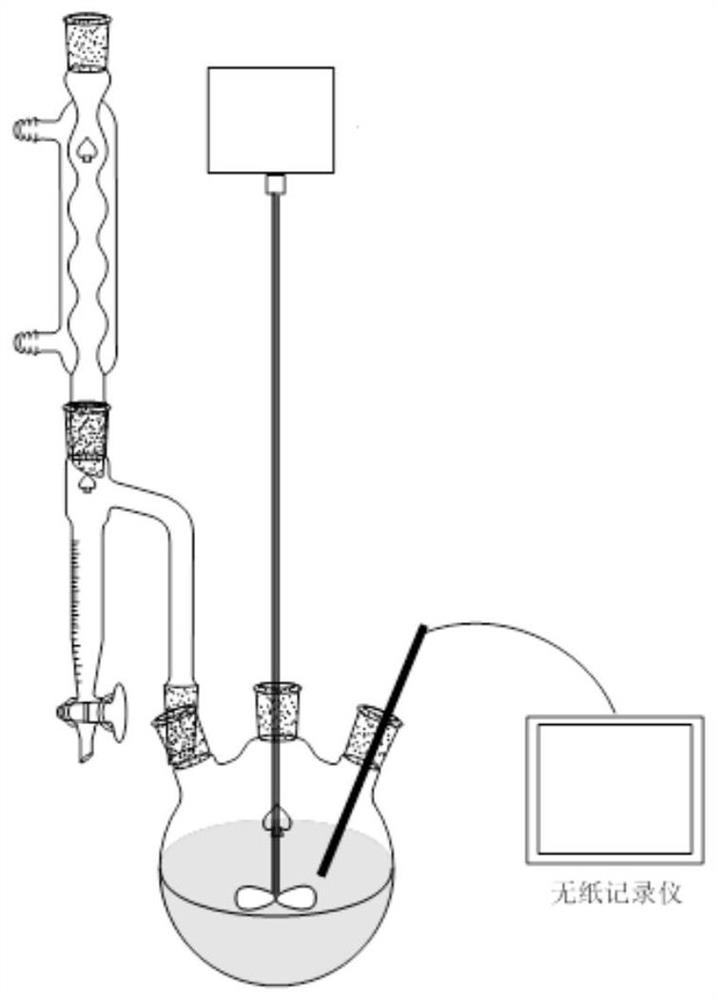
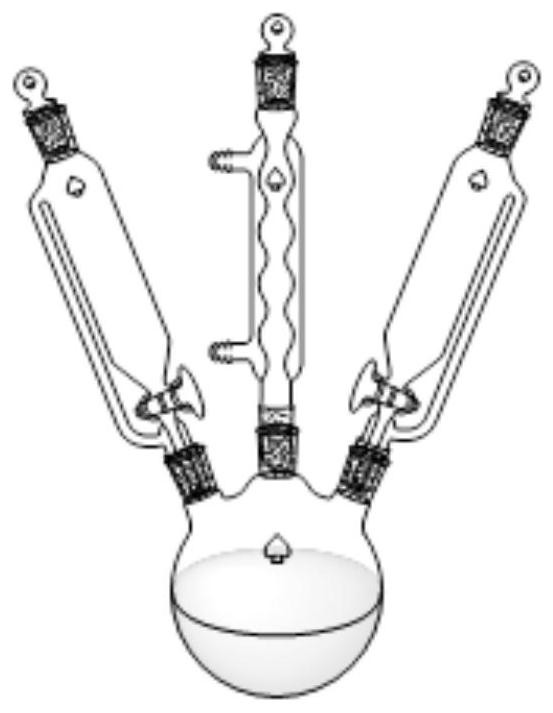
[0069] Get intermediate product K (4.52g, 10mmol) and 50ml acetone and install it in a three-necked flask in a 100ml separating funnel, get tert-butyl hydroperoxide (1.35g, 15mmol) and install it in a three-necked flask in a 100ml separating funnel with 50ml acetone, add Put 50ml of acetone in a three-necked flask, reflux at 90°C, open two separatory funnels, add the material dropwise in 30 minutes, refl...
Embodiment 2
[0072] Synthetic derivatization reagents.
[0073] 4,4-Diphenylethylene dicarboxylic acid (11.3g, 50mmol), hydroquinone (12.1g, 110mmol) and trifluoromethanesulfonic acid (0.37g, 5mmol%) were sequentially added to the reaction tube. Add 50mL of 1,4-dioxane to the reaction bottle, stir and dissolve at room temperature, move the reaction bottle to an oil bath at 60°C and heat to reflux for 2 hours, return the reaction system to room temperature, wash with water, remove the solvent by rotary evaporation, and wash with n-hexane , recrystallized to obtain the intermediate product K in a yield of 86% (19.43 g).
[0074] Get intermediate product K (4.52g, 10mmol) and 50ml1,4-dioxane in a 100ml separatory funnel and install it into a three-necked flask, get tert-butyl hydroperoxide (0.99g, 11mmol) and 50ml1,4-dioxane Install the ring in a 100ml separating funnel to the three-necked flask, add 50ml 1,4-dioxane to the three-necked flask, reflux at 90°C, open the two separating funnels,...
Embodiment 3
[0076] Synthetic derivatization reagents.
[0077] 4,4-diphenylethylene dicarboxylic acid (11.3g, 50mmol), hydroquinone (27.5g, 250mmol) and p-toluenesulfonic acid (4.3g, 50mmol%) were sequentially added into the reaction tube. Add 50mL dimethyl sulfoxide to the reaction bottle, stir and dissolve at room temperature, move the reaction bottle to an oil bath at 60°C and heat to reflux for 2 hours, return the reaction system to room temperature, wash with water, remove the solvent by rotary evaporation, wash with n-hexane, and re- Crystallization afforded intermediate K in 94% (21.24 g) yield.
[0078] Get intermediate product K (4.52g, 10mmol) and 50ml dimethyl sulfoxide in a 100ml separatory funnel and install it into a three-necked flask, get tert-butyl hydroperoxide (1.80g, 20mmol) and 50ml dimethyl sulfoxide in 100ml Install the liquid funnel to the three-necked flask, add 50ml dimethyl sulfoxide to the three-necked flask, reflux at 90°C, open the two separatory funnels, dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com