Application of thio-thiadiazole containing nitrothiazole compound in prevention and treatment of agricultural plant diseases
A technology of nitrothiazole and thiadiazole, which can be used in applications, plant growth regulators, botanical equipment and methods, etc., and can solve problems such as residue toxicity, drug resistance, environmental pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
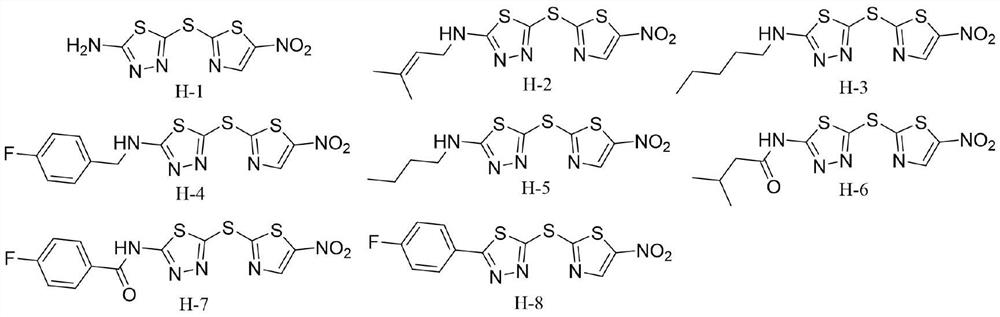
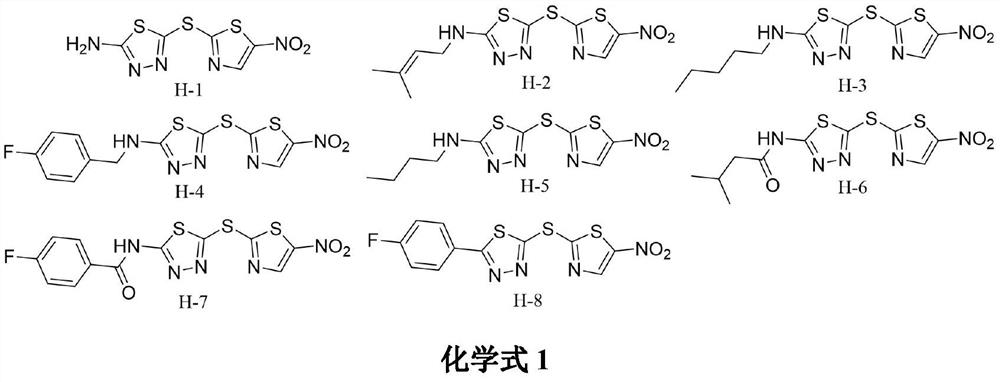
[0011] Embodiment 1: the synthesis of compound H-1
[0012]
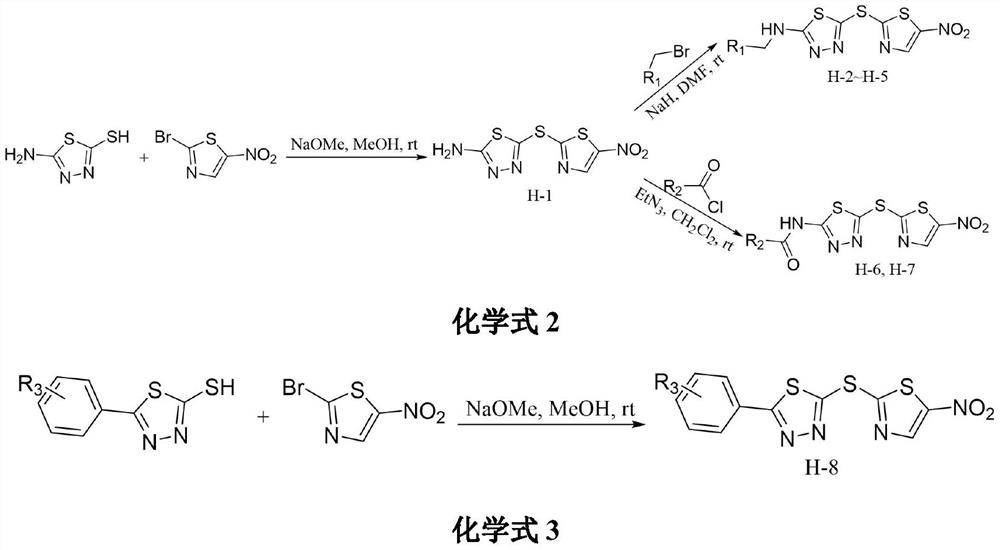
[0013] The synthetic method of compound H-1 of the present invention is carried out according to the following reaction formula:
[0014]
[0015] Synthesis of Compound H-1: Dissolve 5-amino-1,3,4-thiadiazole-2-thiol (1mmol) in methanol (25mL), then add sodium methoxide (1.5mmol) and stir for 10min, then add 2-Bromo-5-nitrothiazole (1mmol), react for 6-7 hours. After the reaction, an appropriate amount of 1 mmol HCl solution was added to the solvent, stirred for 10 min, filtered with suction, and washed three times with a mixed solution of petroleum ether and petroleum ether: ethyl acetate (10:1). The filter residue was collected and dried to obtain the target compound H-1.
[0016] Yield: 66.3%; yellow solid; 1 HNMR (400MHz, DMSO-d 6 )δ:8.74(s,1H),7.94(s,2H). 13 CNMR (100MHz, DMSO-d 6 )δ: 173.51, 171.25, 148.68, 144.45, 140.54. MS-ESI m / z: C 5 h 3 N 5 o 2 S 3 :261.9[M+H] + .
Embodiment 2
[0017] Embodiment 2: the synthesis of compound H-2
[0018]
[0019] The synthetic method of compound H-2 of the present invention is carried out according to the following reaction formula:
[0020]
[0021] Synthesis of Compound H-2: Add Compound H-1 (1mmol) to DMF (15mL) and stir to dissolve under ice bath conditions, then add NaH (3mmol) to the solution and stir for 30min, then slowly add 1-bromo- 3-Methyl-2-butene (1 mmol), react at room temperature for 4 hours. After the reaction was completed, excess saturated ammonium chloride was added to quench the reaction, stirred for 5 min, extracted with dichloromethane, and the organic phases were combined and purified by column chromatography to obtain the target compound H-2.
[0022] Yield: 67.5%; yellow oily liquid; 1 H NMR (400MHz, CDCl 3 )δ: 8.35(s,1H), 5.42–5.28(m,1H), 3.92(d,J=7.8Hz,2H), 1.77(s,3H), 1.76(s,3H). 13 C NMR (100MHz, CDCl 3 )δ: 174.79, 143.51, 140.28, 116.58, 32.36, 25.89, 18.23. MS-ESI m / z: C 10 ...
Embodiment 3
[0023] Embodiment 3: the synthesis of compound H-3
[0024]
[0025] The experimental procedure is the same as in Example 2, except that 1-bromo-3-methyl-2-butene is replaced by 1-bromo-n-hexane. Yield: 64.3%; yellow oily liquid; 1 H NMR (400MHz, CDCl 3 )δ:8.34(s,1H),3.27(t,J=7.4Hz,2H),1.85–1.76(m,2H),1.50–1.31(m,4H),0.92(t,J=7.1Hz,3H ). 13 C NMR (100MHz, CDCl 3 )δ: 175.13, 143.55, 34.07, 30.97, 28.60, 22.25, 14.01. MS-ESI m / z: C 10 h 13 N 5 o 2 S 3 :332.0[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com