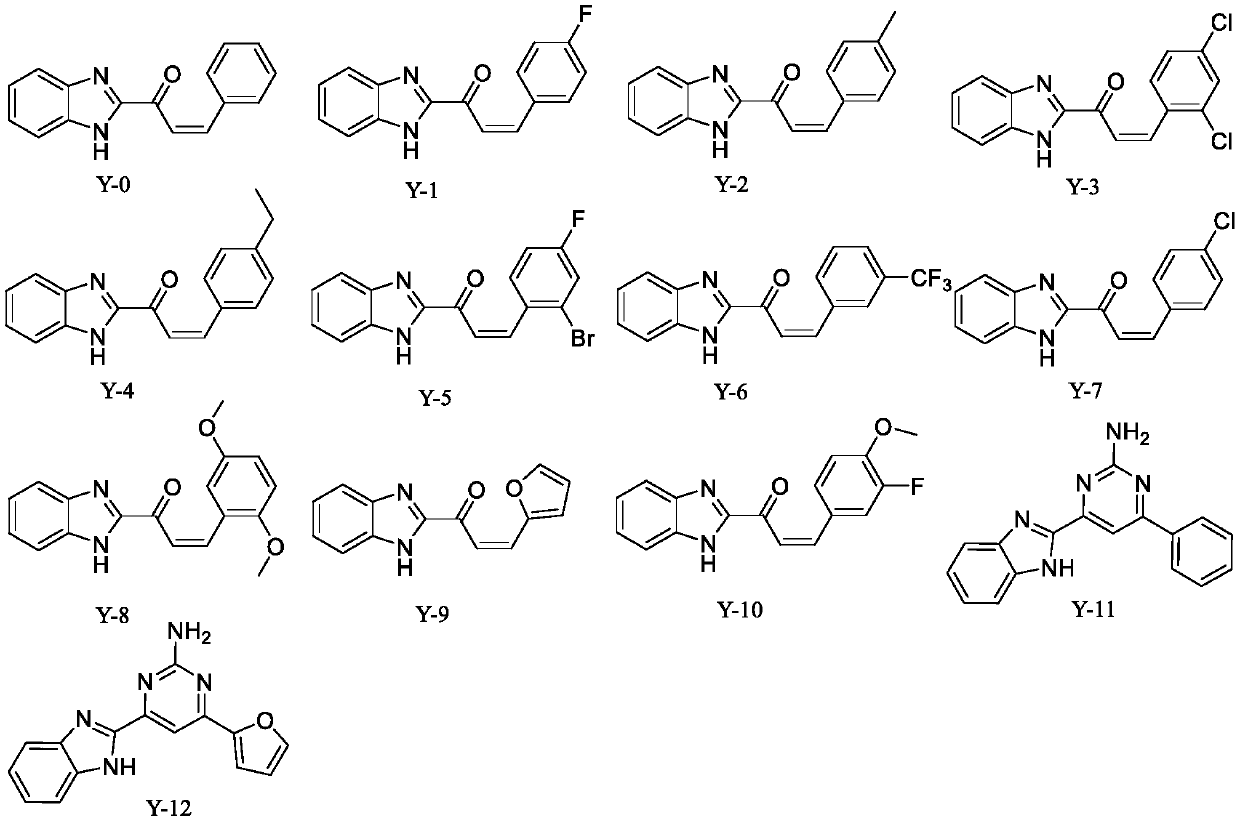
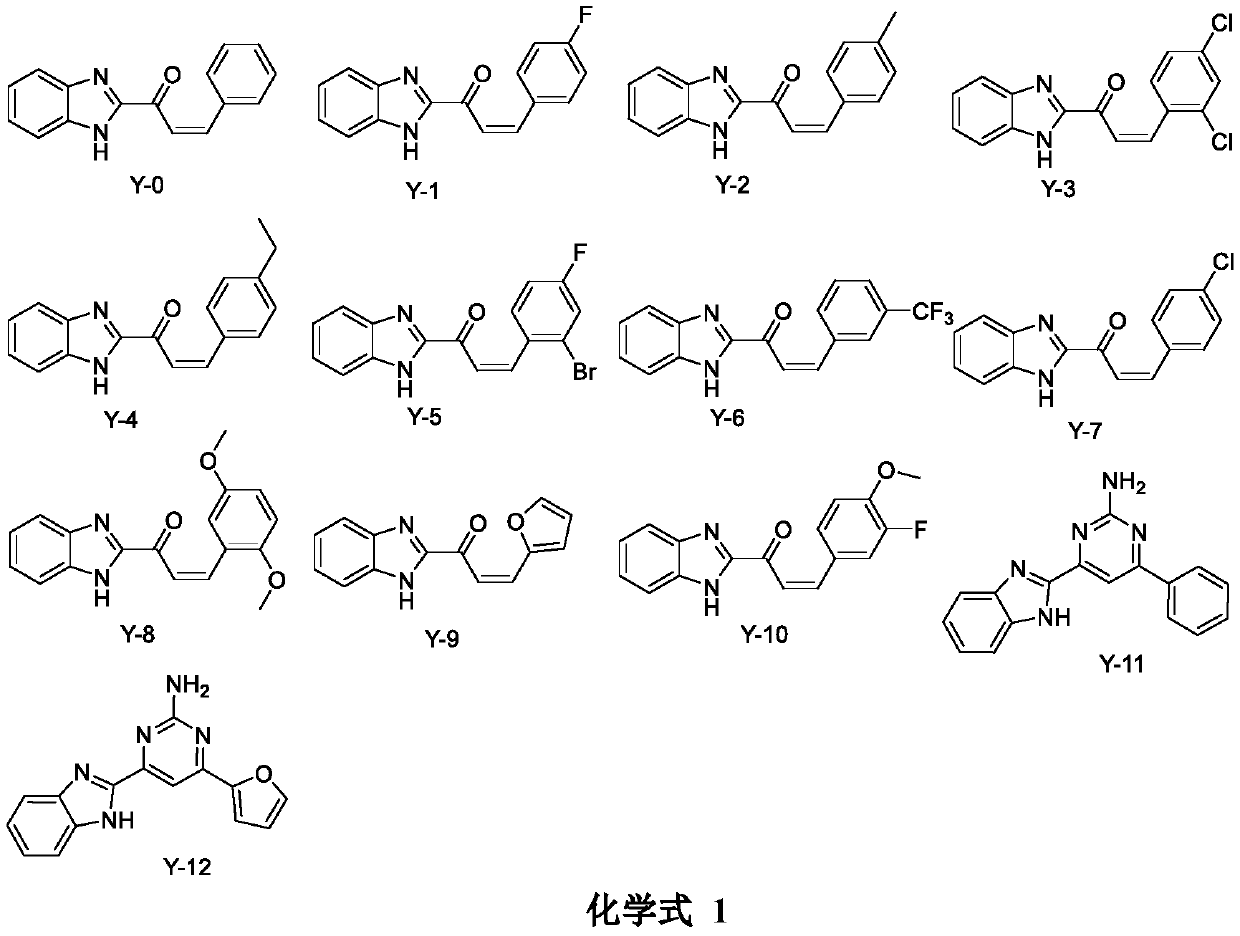
Applications of benzimidazole derivatives in prevention and treatment of agricultural plant diseases
A technology of benzimidazole and its derivatives, applied in the field of medicinal chemistry, can solve problems such as the astonishing growth rate of fungal drug resistance and the single use of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Embodiment 1: the synthesis of compound Y-0
[0012]
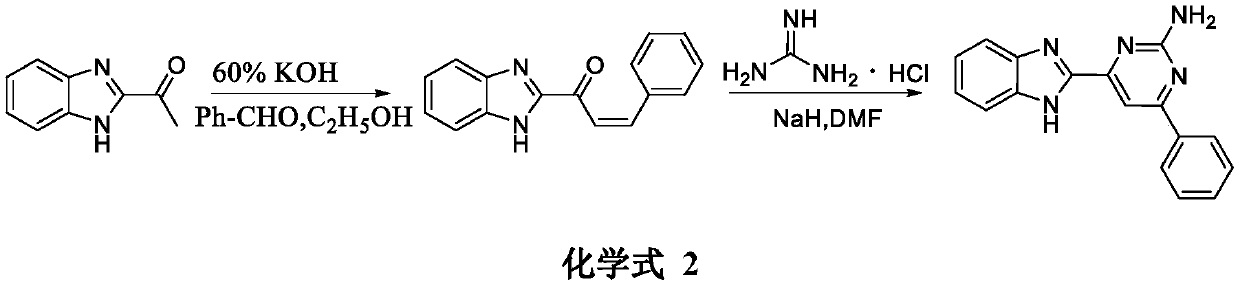
[0013] The synthetic method of compound Y-0 of the present invention is carried out according to the following reaction formula:
[0014]
[0015] Synthesis of the target compound Y-0: at 0°C, add 60% potassium hydroxide aqueous solution (3mL) and benzaldehyde (1mmol) to a solution of 2-acetylbenzimidazole (1mmol) in ethanol (10mL), and Stir until the reaction is complete, and monitor the progress of the reaction by TLC. After the reaction was complete, crushed ice and dilute hydrochloric acid solution were added, the pH was adjusted until a precipitate formed, the crude extract was obtained by filtration and drying, and purified by column chromatography to obtain the target compound Y-0. (Refer to the literature for the synthesis method: European Journal of Medicinal Chemistry 143 (2018) 66-84) Yield: 62.6%; yellow powder; m.p.: 204-205°C; 1 H NMR (400MHz, DMSO-d 6 )δ: 7.89(d, J=6.8Hz, 1H), 7.68(d, J=8.4Hz...
Embodiment 2
[0016] Example 2: Synthesis of Compound Y-1 The synthesis method is the same as in Example 1, except that benzaldehyde is replaced by 4-fluorobenzaldehyde.
[0017]
[0018] Yield: 72.1%; light yellow powder; m.p.: 200-201°C; 1 H NMR (400MHz, DMSO-d 6 )δ: 8.38(d, J=9.6Hz, 1H), 8.01(d, J=8.1Hz, 2H), 7.52(t, J=7.9Hz, 2H), 7.34–7.27(m, 2H), 7.24( s, 2H), 7.10(d, J=7.9Hz, 1H), 6.94(d, J=8.5Hz, 1H), 6.20(d, J=4.1Hz, 1H). 13 C NMR (100MHz, DMSO-d 6 )δ: 181.20, 162.43, 145.66, 141.40, 137.29, 135.76, 132.28, 131.52, 131.36, 123.32, 123.06, 121.04, 121.82, 120.96, 117.34, 115.62, 112.38 / z: ms-ci 16 h 11 FN 2 O: 267.7 [M+H] +
Embodiment 3
[0019] Embodiment 3: the synthesis of compound Y-2
[0020] The synthetic method is the same as that of Example 1, except that benzaldehyde is replaced with 4-methylbenzaldehyde.
[0021]
[0022] Yield: 68.8%; orange powder; m.p.: 200.0-201°C; 1 H NMR (400MHz, DMSO-d 6 )δ: 13.53(s, 1H), 8.09(d, J=16.1Hz, 1H), 7.97(d, J=16.0Hz, 1H), 7.78(d, J=7.5Hz, 4H), 7.39(s, 2H), 7.32(d, J=7.4Hz, 2H), 2.37(s, 3H); 13 C NMR (100MHz, DMSO-d 6 )δ: 181.39, 149.52, 144.78, 143.51, 141.79, 132.07, 130.24, 130.24, 129.43, 129.43, 126.13, 123.62, 121.61, 121.01, 113.34, 21.59. MS-ESI m / z: C 17 h 14 N 2 O: 263.1 [M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com