Fosaprepitant composition and preparation method thereof
A technology of fosaprepitant dimeglumine and composition, which is applied in the direction of drug combination, non-active ingredients of polymer compounds, medical preparations of non-active ingredients, etc., and can solve life-threatening problems
Pending Publication Date: 2021-01-19
SPES PHARMA INC
View PDF8 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
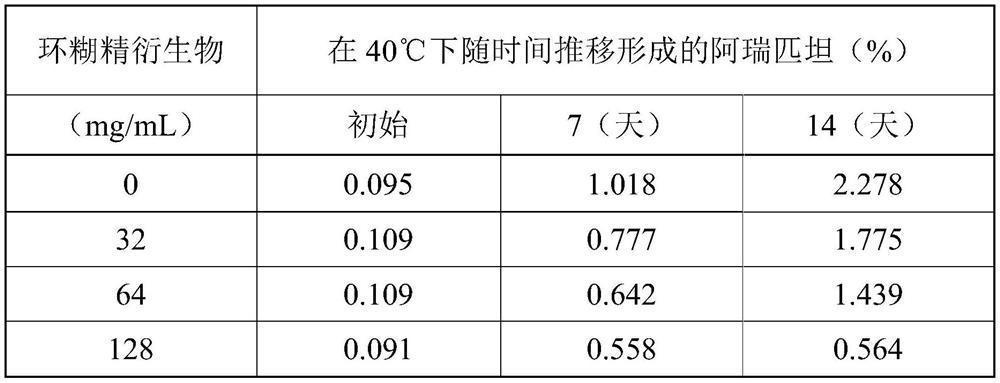
However, polysorbate 80, a common solubilizer for low solubility drug formulations, is known to cause a variety of adverse reactions, including severe infusion site reactions and potentially life-threatening anaphylaxis
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
example 1
[0057]
example 2
[0059]
[0060]
example 3
[0062]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Login to View More
Abstract
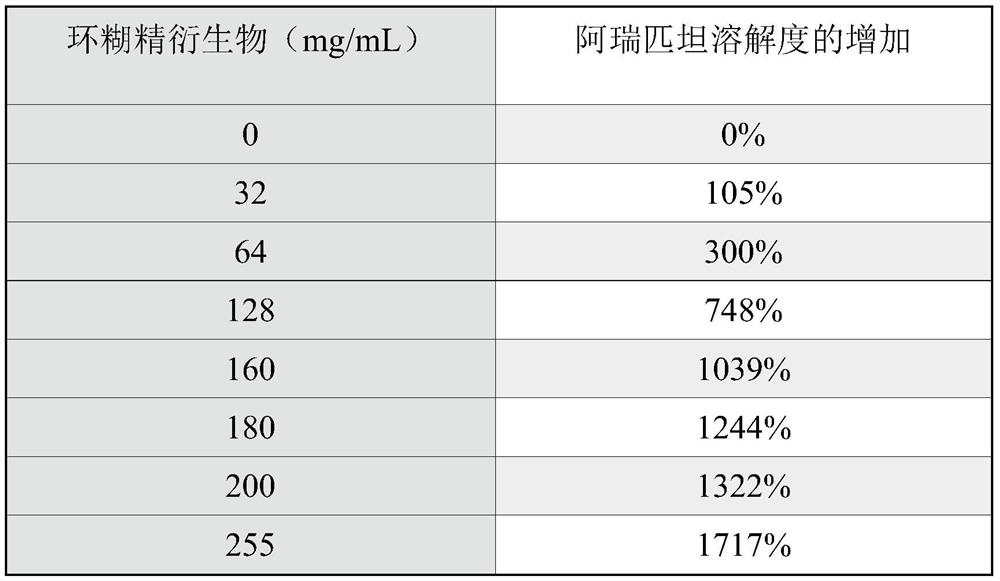
The present application and its embodiments teach stable compositions of fosaprepitant or a pharmaceutically acceptable salt thereof with such compositions lacking polysorbate 80 and containing dual functional excipients of hydrolysis inhibition and solubility enhancement. Further described are methods of preparation of such compositions. Among other advantages of contemplated compositions, fosaprepitant hydrolysis degradation is kept low and the compositions maintain physically and chemically stable for prolonged period.
Description
[0001] priority claim [0002] This application claims priority to U.S. Application No. 16 / 245,992, filed January 11, 2019, which claims priority to U.S. Provisional Application No. 62 / 673,522, filed May 18, 2018 Right, the entire content of said application is incorporated herein by reference in its entirety. technical field [0003] The field of the invention and its embodiments relates to stable compositions of fosaprepitant or a pharmaceutically acceptable salt thereof. In particular, the present application relates to compositions of fosaprepitant dimeglumine that do not contain polysorbate 80 or similar surfactants. Methods of preparing such compositions are further described herein. Background technique [0004] Fosaprepitant dimeglumine is an injectable drug sold by Merck Sharp & Dohme Corp. active pharmaceutical ingredients. Fosaprepitant is a water-soluble phosphorylated prodrug of aprepitant that is rapidly converted to aprepitant in vivo after intravenous (I...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/675A61K47/40A61K9/08A61P1/08
CPCA61K31/675A61K9/0019A61K47/183A61K47/40A61K47/02A61K9/08
Inventor 虞建伟王玉露陶奇海
Owner SPES PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com