Rabbit-derived paraoxonase 1 mutant and recombinant expression method thereof
A paraoxonase and mutant technology, applied in the field of rabbit paraoxonase 1 mutant and its recombinant expression, can solve the problem of single genetic background, lack of protein post-translational modification system, large difference in codon preference, etc. problem, to achieve the effect of efficient recombinant expression
Inactive Publication Date: 2021-01-15
北京森根比亚生物工程技术有限公司
View PDF1 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0004] The Escherichia coli expression system is simple to operate, has a short cycle, a single genetic background, low cost, and easy purification of expression products; however, for serum paraoxonase PON1 derived from mammals, due to the lack of a protein post-translational modification system in Escherichia coli, both Escherichia coli and The codon preference of mammals varies greatly, so it is difficult to express soluble and active rePON1 protein in E. coli
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
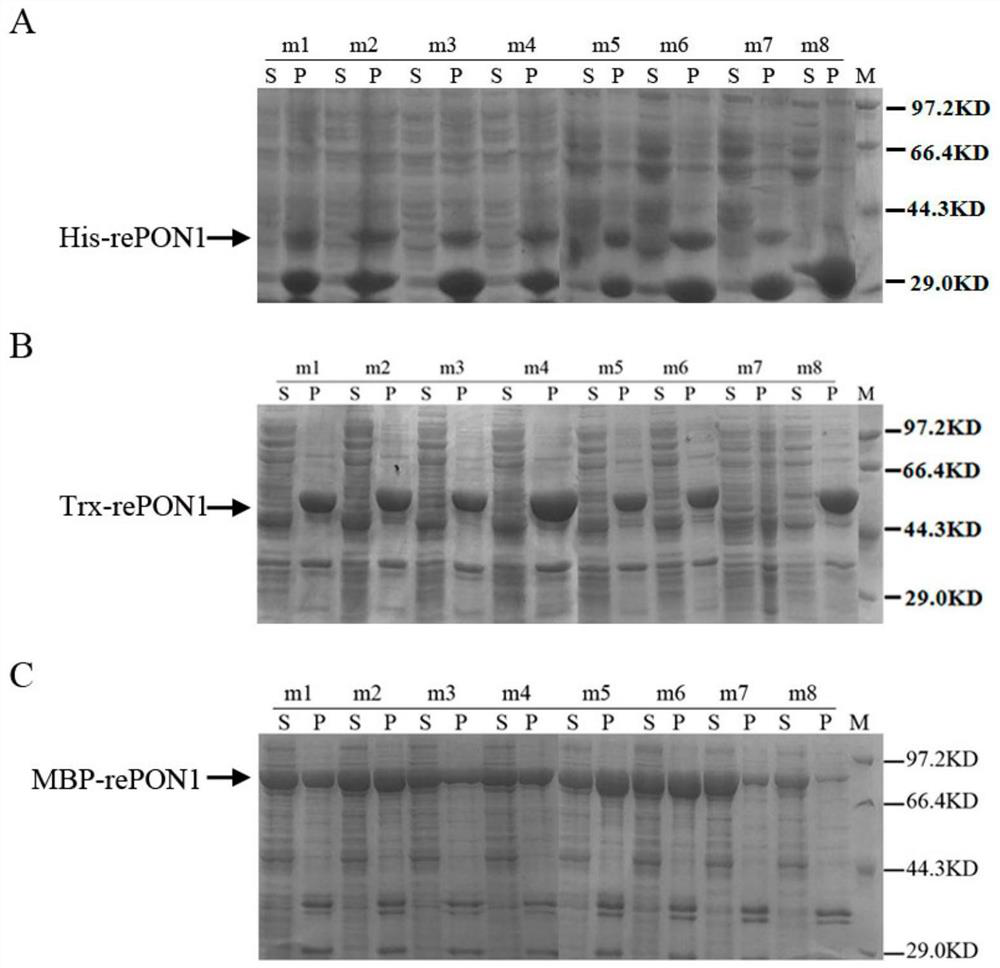
[0041] Embodiment 1 mutant gene cloning and protein induced expression, recombinant protein enzymatic performance measurement
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
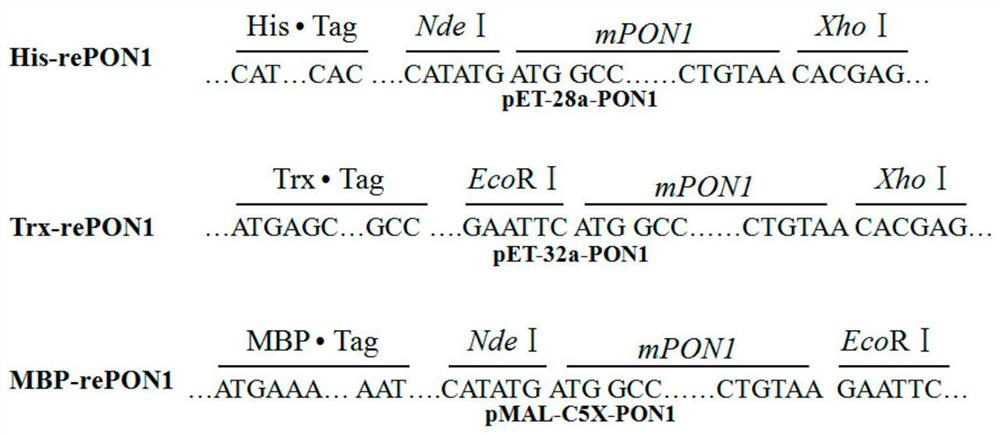
The invention discloses a rabbit-derived paraoxonase 1 mutant and a recombinant expression method thereof. The method comprises the following steps: cloning and isolating the PON1 gene of a Japanese white rabbit; carrying out genetic engineering modification to obtain a plurality of point mutation genes; carrying out codon optimization on each mutation gene according to the codon preference of escherichia coli, constructing prokaryotic expression vectors carrying different labels, carrying out induced expression, and carrying out screening to obtain Trx-rePON1 and MBP-rePON1 proteins. The mutant can generate complete and active soluble protein in an escherichia coli expression system, has strong hydrolytic performance on para-phenyl acetate and paraoxon-ethyl, and has the hydrolytic activity on the paraoxon-ethyl being respectively 5 times and 19 times of the activity of rabbit-derived background serum PON1 and the activity of recombinant expression PON1 in bombyx mori. According to the method, efficient recombinant expression of rabbit-derived PON1 in escherichia coli can be achieved, and a new choice is provided to application of PON1 in the fields of organophosphorus degradationand biomedicine.
Description
technical field [0001] The present invention relates to a paraoxonase 1 mutant, in particular to a rabbit-derived paraoxonase 1 mutant and its encoding gene and its recombinant expression method in a prokaryotic expression system, belonging to a rabbit-derived paraoxonase 1 mutant and its Recombine the expression domain. Background technique [0002] PON1 (Paraoxonase1) paraoxonase 1, enzymatic classification EC3.1.8.1, belongs to the lactonase family. Since the 1950s, paraoxonase PON1 derived from mammalian serum has been continuously isolated, and PON1 and homologous genes have been cloned from rabbits, mice, cattle and humans (Mackness, M.and B. Mackness, Humanparaoxonase-1 (PON1): Gene structure and expression, promiscuous activities and multiple physiological roles. Gene, 2015.567(1): p.12-21.). Paraoxonase is a natural barrier for mammals, especially humans, to protect and detoxify. It plays a very important role in protecting the body from organophosphate damage. It...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C12N15/55C12N9/16C12N15/70C12N1/21A61K31/7088A61K38/46A61P39/02C12R1/19
CPCC12N9/16C12Y301/08001C12N15/70A61K31/7088A61P39/02C12N2800/22A61K38/00
Inventor 龚晓洁王璋
Owner 北京森根比亚生物工程技术有限公司
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com