Pseudosciaena crocea Cystatin recombinant protein and application thereof
A technology of recombinant protein and large yellow croaker, applied in the field of genetic engineering, can solve the problems of lack of applied research and achieve the effect of enhancing the antibacterial function of macrophages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1 Construction of Pichia pastoris engineering bacteria that highly express recombinant protein Cystatin of large yellow croaker
[0015] The large yellow croaker Cystatin recombinant protein of the present invention is the mature peptide sequence of large yellow croaker Cystatin (amino acid 20-138, as shown in SEQ ID NO.2), and the sequence of large yellow croaker Cystatin has been published (Genbank: XM_027278065.1). Using the spleen cDNA of large yellow croaker as a template, use the primers Cystatin-F and Cystatin-R to amplify the gene fragment encoding the mature peptide of large yellow croaker Cystatin (sequence shown in SEQ ID NO.1), and then use the pEASY- Uni Seamless Cloning and Assembly Kit connects the target fragment to the yeast expression vector pPICZαA to obtain the recombinant expression vector pPICZαA-Cystatin. Recombinant vector pPICZαA-Cystatin was linearized by Mss I digestion, and transformed into Pichia pastoris SMD1168 competent cells by e...
Embodiment 2
[0018] Embodiment 2 Cystatin recombinant protein expression and purification of large yellow croaker
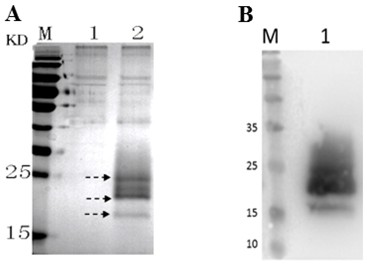
[0019] (1) Expression analysis of Cystatin recombinant protein in large yellow croaker
[0020] The Pichia pastoris that embodiment 1 obtains Pichia pastoris SMD1168 / pPICZαA-Cystatin engineered bacteria were inoculated into yeast medium 1, and cultured with shaking at 30 °C and 220 rpm. When the OD600 reaches 2.0, pour the above bacterial solution into a centrifuge tube, centrifuge at 6000 g at 4 °C for 7 min, remove the supernatant, resuspend the bacterial cells with yeast medium 2, and culture with shaking at 30 °C, adding 1 % methanol, induce expression for 4 days, and then use SDS-PAGE to analyze the expression of Cystatin recombinant protein in large yellow croaker in the culture supernatant. The results showed that compared with the control strain, Pichia pastoris Pichia pastoris In the culture supernatant of SMD1168 / pPICZαA-Cystatin engineering bacteria, the targe...
Embodiment 3
[0027] Inhibitory activity of embodiment 3 large yellow croaker Cystatin recombinant protein
[0028] (1) Inhibitory activity on papain
[0029]In a 100 µL reaction system, add 20 µL papain (20 nM), 10 µL 10×papain buffer, 40 µLddH 2 O, then add 10 µL of Cystatin recombinant protein from large yellow croaker with a final concentration of 0 nM, 5 nM, 10 nM, 20 nM and 25 nM, and finally mix with 20 µL of the substrate Z-FR-AMC with a final concentration of 12.5 µM , under the conditions of excitation wavelength of 380 nm and emission wavelength of 460 nm, the fluorescence released by hydrolysis of Z-FR-AMC was measured every 30 s using a multi-functional microplate reader SpectraMaxM5. The results showed that the Cystatin recombinant protein of large yellow croaker had obvious inhibitory effect on the activity of papain, and the inhibition constant was 3.012×10 -11 M( figure 2 A);
[0030] The 10×papain buffer formulation is: 4 M sodium phosphate, 40 mM EDTA, 40 mM DTT, pH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com