Method for determining hydroxytyrosol in Beagle dog plasma
A technology of hydroxytyrosol and plasma, applied in the field of determination of hydroxytyrosol in Beagle dog plasma, can solve the problems of poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
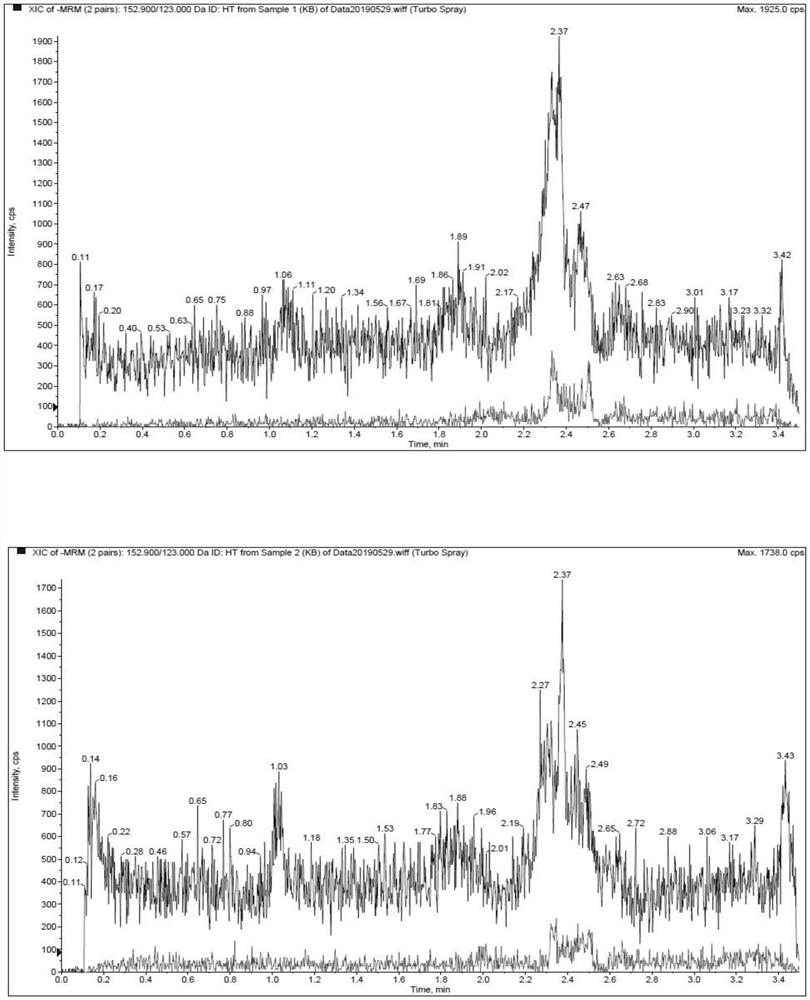
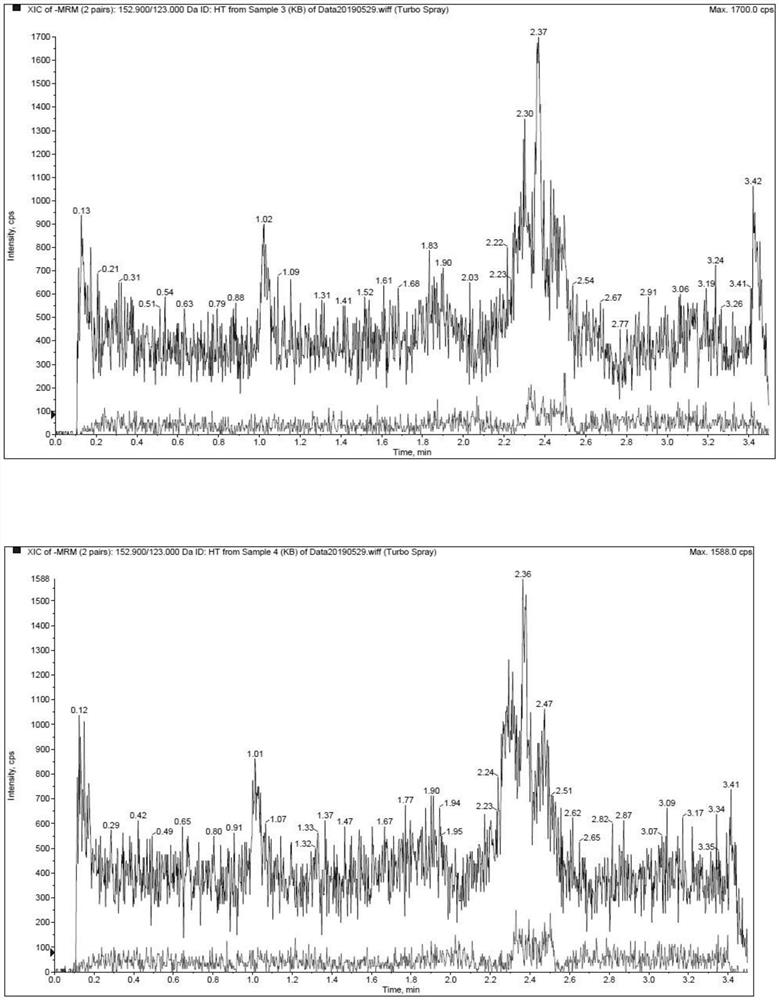
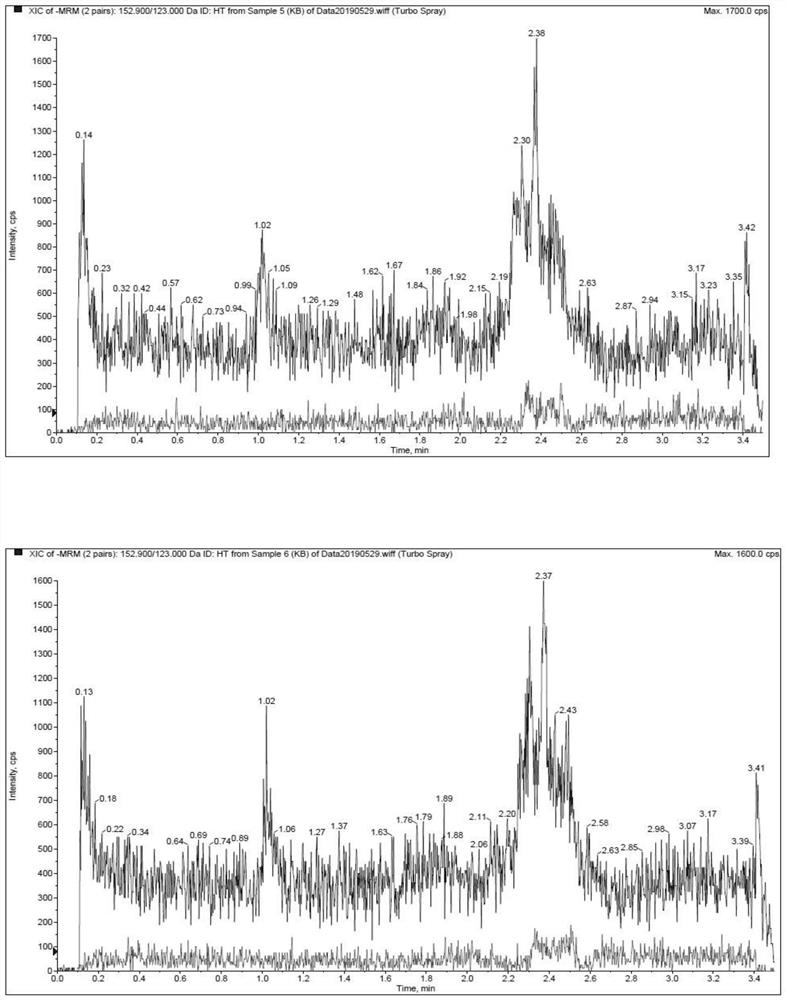
[0022] The methodological investigation of embodiment 1 detection method of the present invention
[0023] 1. Preparation of standard solution
[0024] (1) Preparation of standard series solutions
[0025] Accurately weigh an appropriate amount of hydroxytyrosol, add solvent (containing 0.1% formic acid, 5mM ammonium acetate, 5mM ascorbic acid aqueous solution) and quantitatively dilute to prepare a 4mg / mL standard stock solution. Precisely measure an appropriate amount of standard stock solution, dilute it with a solvent gradient, and prepare a series of standard solutions with concentrations of 10, 20, 50, 200, 500, 2000, 5000, 10000, and 20000 ng / mL.
[0026] (2) Preparation of internal standard solution
[0027] Accurately weigh an appropriate amount of tolbutamide, add acetonitrile to dissolve and quantitatively dilute to prepare a 10 mg / mL tolbutamide stock solution. Precisely measure an appropriate amount of stock solution, and gradually dilute it with acetonitrile t...
Embodiment 2
[0052] Embodiment 2 Hydroxytyrosol pharmacokinetic experiment in Beagle dog body
[0053] The experiment adopted a two-period crossover design. 4 Beagle dogs, male, were randomly divided into 2 groups. After fasting for 12 hours, the intravenous injection group was given hydroxytyrosol solution by intravenous injection, and the oral group was given hydroxytyrosol capsules orally at a dosage of 14 mg / kg. About 1.5 mL of blood was collected from the canine forelimb vein in each group at 0.083, 0.167, 0.333, 0.5, 1, 2, 4, 6, 8 and 24 hours after administration.
[0054] The blood samples were placed on ice after collection, and the plasma was centrifuged within 1 hour (centrifugation conditions: 8000 rpm, 10 min, 2-8°C). After 14 days, the animals of the two groups were cross-administered, and the venous blood was collected and the plasma was prepared according to the above method. The collected plasma was stored in a -80°C refrigerator before analysis.
[0055] The determina...
PUM
| Property | Measurement | Unit |
|---|---|---|
| collision gas | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com