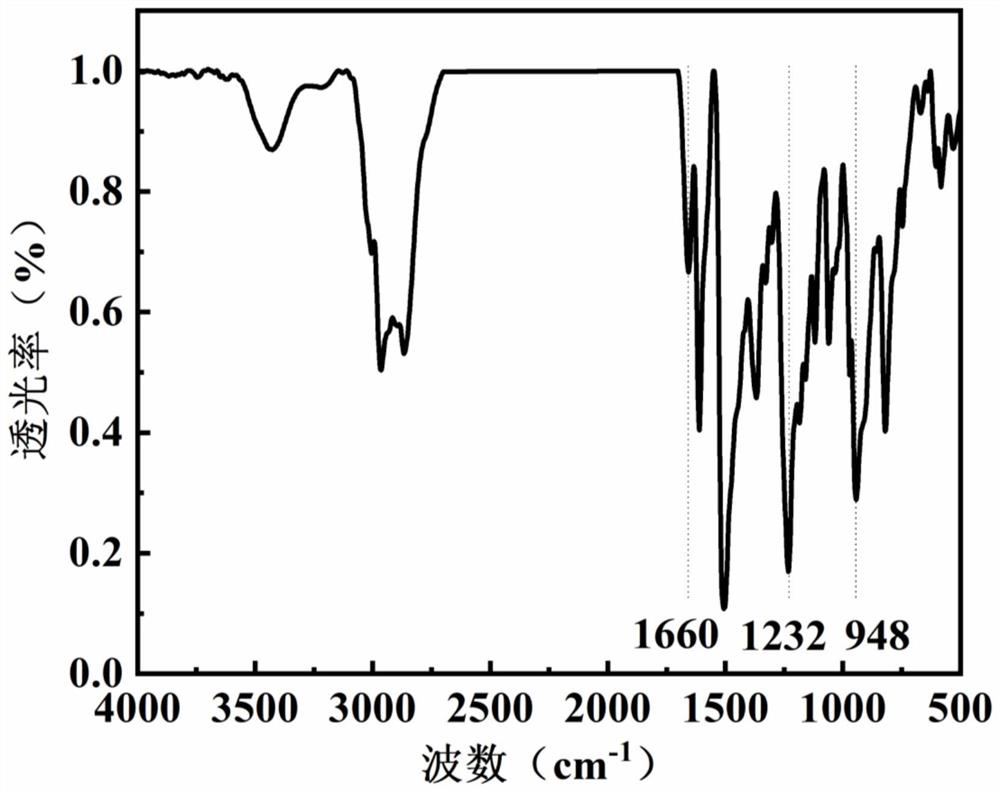
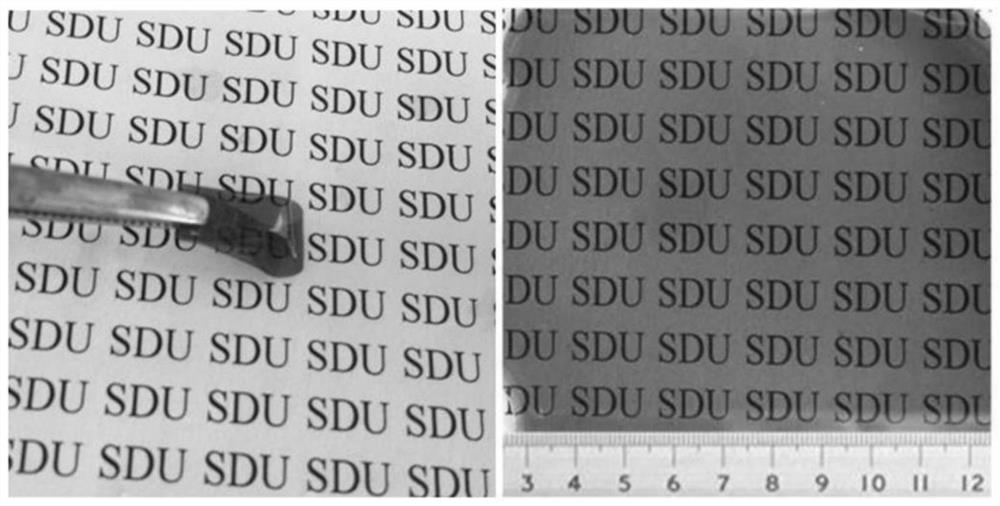
Benzoxazine resin containing quaternary ammonium group and preparation method and application of benzoxazine resin
A technology of quaternary ammonium group and benzoxazine, applied in the field of main chain type benzoxazine resin and its preparation, can solve problems such as high price and reduced conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1 Synthesis of quaternary ammonium group-containing main chain type benzoxazine based on bisphenol A and 4,4'-diaminodiphenylmethane
[0105] Dissolve 1.1g of bisphenol A, 2.0g of 4,4'-diaminodiphenylmethane, 1.6g of 4-(2-dimethylaminoethyl)phenol, 1.4g of formaldehyde and 0.2g of triethylamine in 30ml of dimethyl In sulfoxide, the temperature was raised to 90°C for 5h. Post-processing: remove the solvent under reduced pressure, wash with methanol, ethanol, and n-hexane in turn, and dry in vacuum at 70°C overnight to obtain the main chain type benzoxazine.
[0106] 1.1 g of main chain type benzoxazine and 0.3 g of methyl iodide were dissolved in 20 mL of dioxane, heated to 80° C. under magnetic stirring and reacted for 3 hours. After the reaction is finished, filter and vacuum-dry the filter cake to obtain a main-chain benzoxazine containing quaternary ammonium groups with a yield of 95%.
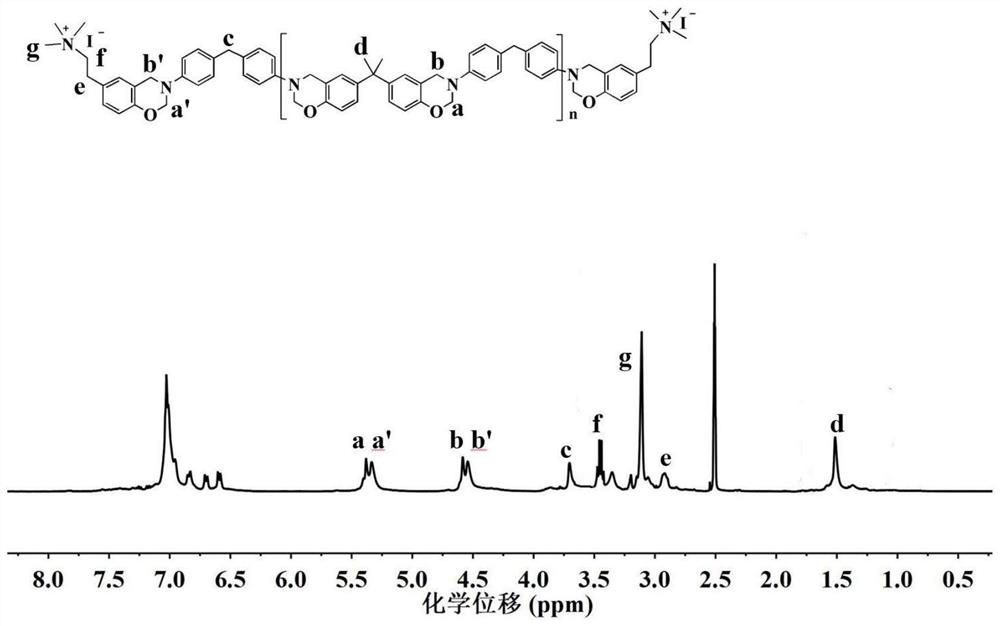
[0107] 1 H NMR (400MHz, d 6 -DMSO, ppm): δ=5.34 (d, J=17.6Hz, O-CH ...
Embodiment 2
[0109] Example 2 Synthesis of quaternary ammonium group-containing main chain type benzoxazine based on bisphenol S and 4,4'-diaminodiphenyl ether
[0110] Dissolve 1.5g of bisphenol S, 2.4g of 4,4'-diaminodiphenyl ether, 1.9g of 4-dimethylaminomethylphenol, 1.8g of formaldehyde and 0.3g of pyridine in 30mL of ethanol, heat up to 85°C for 12 hours . Post-processing: remove the solvent under reduced pressure, wash with methanol, ethanol, and n-hexane in turn, and dry in vacuum at 50°C overnight to obtain the main chain type benzoxazine.
[0111] Dissolve 1.5 g of main-chain benzoxazine and 0.6 g of bromoethane in 15 mL of dimethyl sulfoxide, raise the temperature to 90° C. under magnetic stirring, and react for 5 hours. After the reaction, filter and vacuum-dry the filter cake to obtain the main chain type benzoxazine containing quaternary ammonium groups, with a yield of 90%.
Embodiment 3
[0112] Example 3 Synthesis of quaternary ammonium group-containing main chain type benzoxazine based on bisphenol AF and 4,4'-diaminodiphenylsulfone
[0113] Dissolve 1.8g of bisphenol AF, 2.0g of 4,4'-diaminodiphenylsulfone, 1.9g of 4-dimethylaminomethylphenol, 1.8g of formaldehyde and 0.5g of triethylamine in 30mL of toluene, heat up to 90°C for reaction 20h. Post-processing: remove the solvent under reduced pressure, wash with methanol, ethanol, and n-hexane in turn, and dry in vacuum at 60° C. overnight to obtain the main chain type benzoxazine.
[0114] Dissolve 1.9 g of main-chain benzoxazine and 0.9 g of diethyl sulfate in 25 mL of N,N-dimethylformamide, raise the temperature to 90° C. under magnetic stirring, and react for 5 hours. After the reaction, filter and vacuum-dry the filter cake to obtain main-chain benzoxazine containing quaternary ammonium groups, with a yield of 84%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com