Soluble drug-loaded microneedle patch for treating recurrent aphthous ulcer as well as preparation method and application of soluble drug-loaded microneedle patch
A micro-needle sticking and dissolving technology, which is applied in the field of biomedical materials, can solve the problems of complex, difficult to change, and fixed size of the micro-needle negative mold, and achieves the improvement of local effective drug concentration, the preparation method is simple and clear, and the size is controllable. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: 3D printing microneedle negative mold
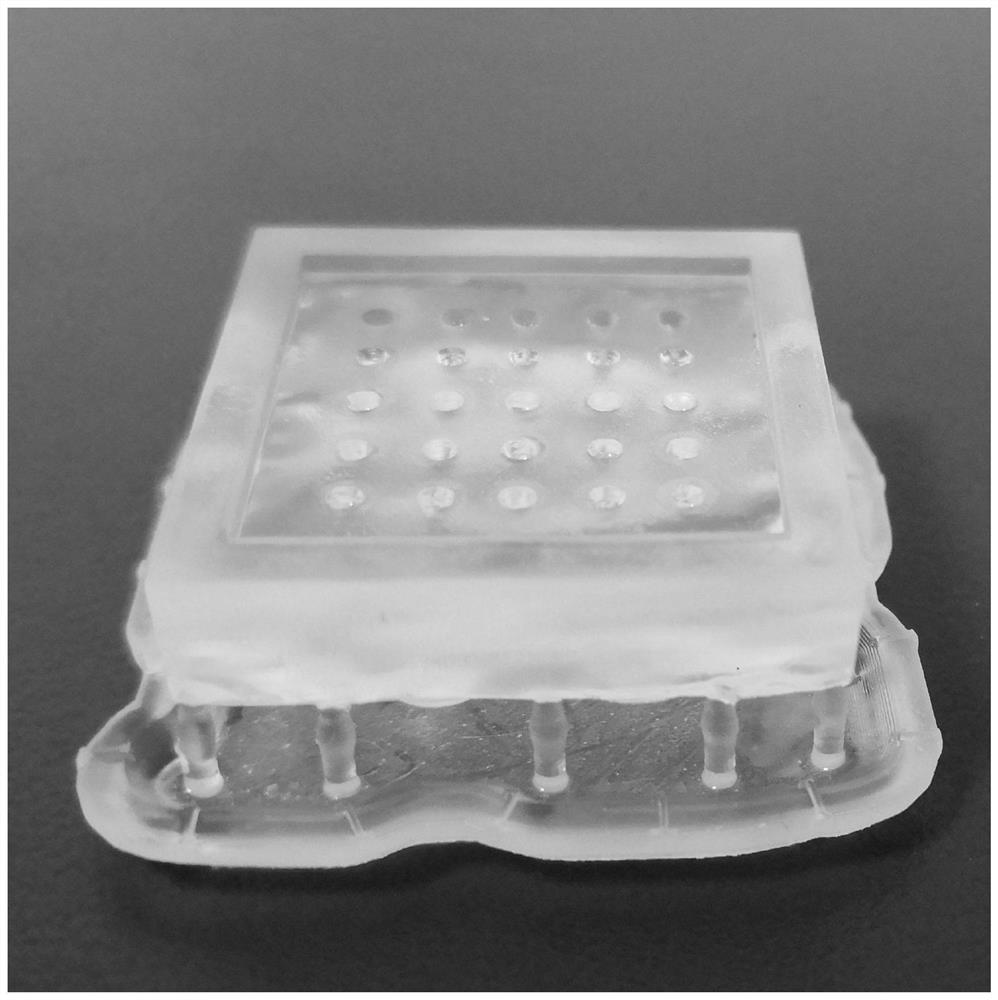
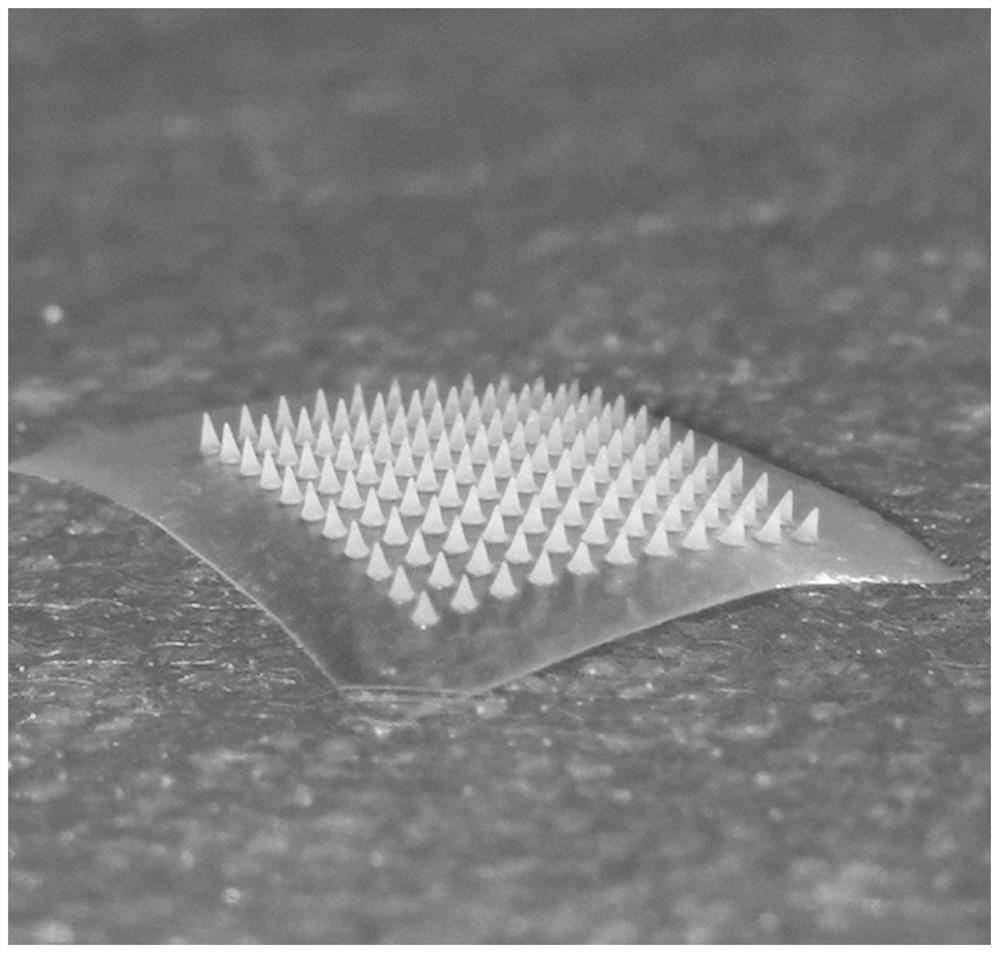
[0026] S1, through 3DMax design the microneedle negative mold model that meets the requirements of the present invention. The microneedle negative mold used in the present invention is a conical microneedle with a diameter of 0.75 mm and a height of 3 mm, and is saved in an STL format file.
[0027] S2, import the STL file into the light-curing 3D printer, and use the 3D printed light-curing resin to print, and the thickness of the slice is 0.05mm.
[0028] S3, after the printing is completed, soak the model with isopropanol solution for 10 minutes to wash away excess resin and obtain a negative microneedle model.
Embodiment 2
[0029] Example 2 Preparation of soluble drug-loaded hyaluronic acid microneedle patch
[0030] S1, using the 3D printed microneedle template in Example 1;
[0031] S2, preparation of hyaluronic acid substrate preparation solution (10%, w / v): Weigh 1 g of sodium hyaluronate (molecular weight 40,000-100,000) and dissolve it in 9 mL of deionized water, and stir for 24 hours under magnetic stirring to ensure sufficient swelling;
[0032] S31, the weight ratio of bovine basic fibroblast growth factor, cetylpyridinium chloride and hyaluronic acid substrate preparation solution in the needle body preparation solution is (0.08~0.1)×10 -5 : 0.05~0.1:1 onto the microneedle template in step S1, and centrifuged in a 10mL centrifuge tube at 3000rpm for 1h, so that the microneedle template needle tip preparation solution fills the pinhole;
[0033]S32, placing the microneedle template containing the needle tip preparation solution in step S31 in a vacuum drying oven, adjusting the air pres...
Embodiment 3
[0039] Example 3 Preparation of soluble drug-loaded sodium alginate microneedle patch
[0040] S1, using the 3D printed microneedle template in Example 1;
[0041] S2, preparation of sodium alginate substrate preparation solution (5%, w / v): Weigh 0.5 g of sodium alginate and dissolve it in 9.5 mL of deionized water, stir magnetically at 50 °C for 24 hours to ensure sufficient swelling;
[0042] S31, the weight ratio of recombinant human epidermal growth factor, compound chlorhexidine, and sodium alginate substrate preparation solution in the preparation solution of the needle body is (0.05-0.15)×10 -5 : 0.05~0.1:1 onto the microneedle template in step S1, and centrifuged in a 10mL centrifuge tube at 3000rpm for 1h, so that the microneedle template needle tip preparation solution fills the pinhole;
[0043] S32, placing the microneedle template containing the needle tip preparation solution in step S31 in a vacuum drying oven, adjusting the air pressure to 0.1Pa, drying at 37°...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com