Application of fangchinoline in preparation of medicine for preventing and treating ulcerative colitis
A technology for ulcerative colitis and fangchinoline, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Experimental materials
[0028] 1.1 Medicinal materials
[0029] Fangchinoline base (CAS: 436-77-1, MW 608.71, HPLC purity ≥ 98%, Chengdu Alpha Biotechnology Co., Ltd.); Dextran sodium sulfate (DSS, CAS: 9011-18-1, MW 36- 50kDa, produced by MP Biomedicals, USA); sulfasalazine (SASP, CAS: 599-79-1, molecular weight 398.39, HPLC purity ≥ 98%, produced by Sigma-Aldrich, USA).
[0030] 1.2 Experimental animals
[0031] C57BL / 6J female mice (8 weeks) weighing 20±2 g were provided by the Experimental Animal Center of Shanghai University of Traditional Chinese Medicine, animal certificate number: SYXK (Shanghai) 2018-0032. Placed in a conventional feeding environment, free access to food and water.
[0032] 2. Experimental method
[0033] 2.1 Establishment of ulcerative colitis model
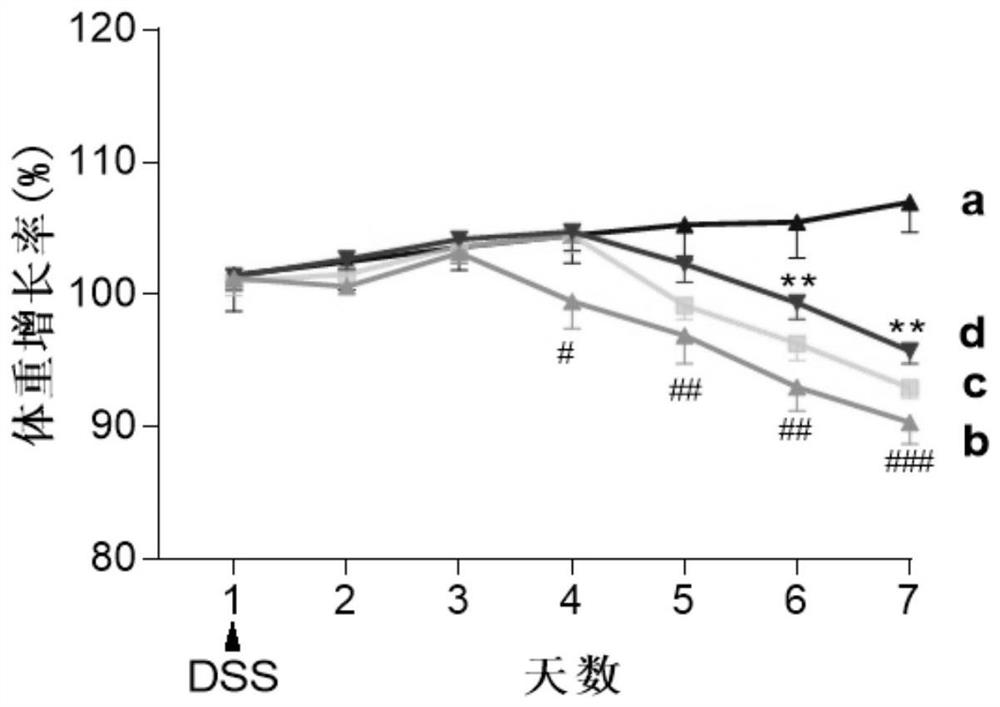
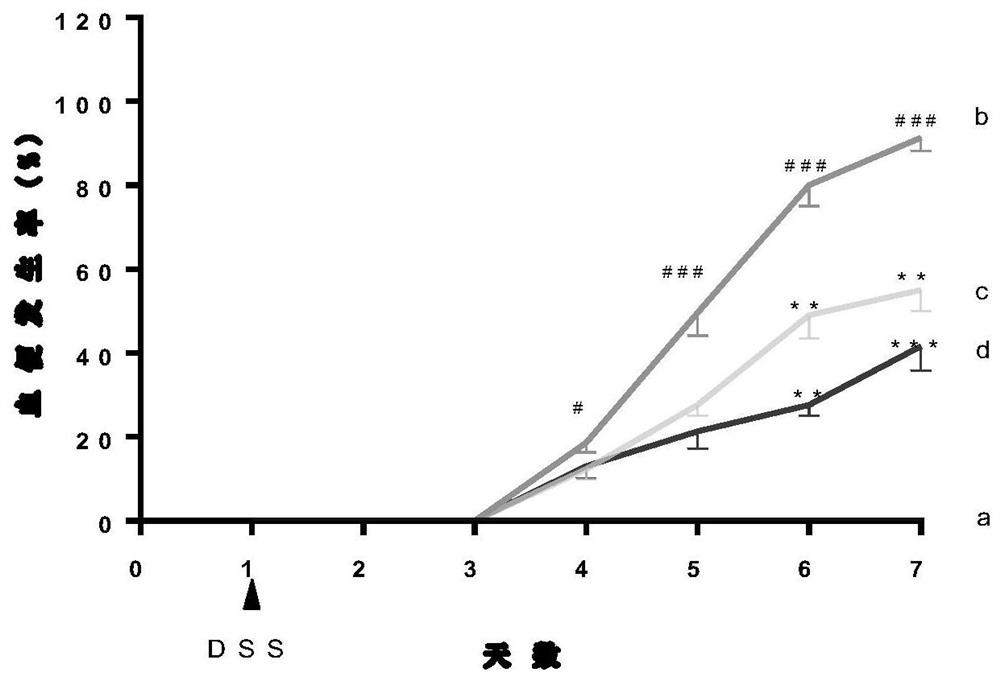
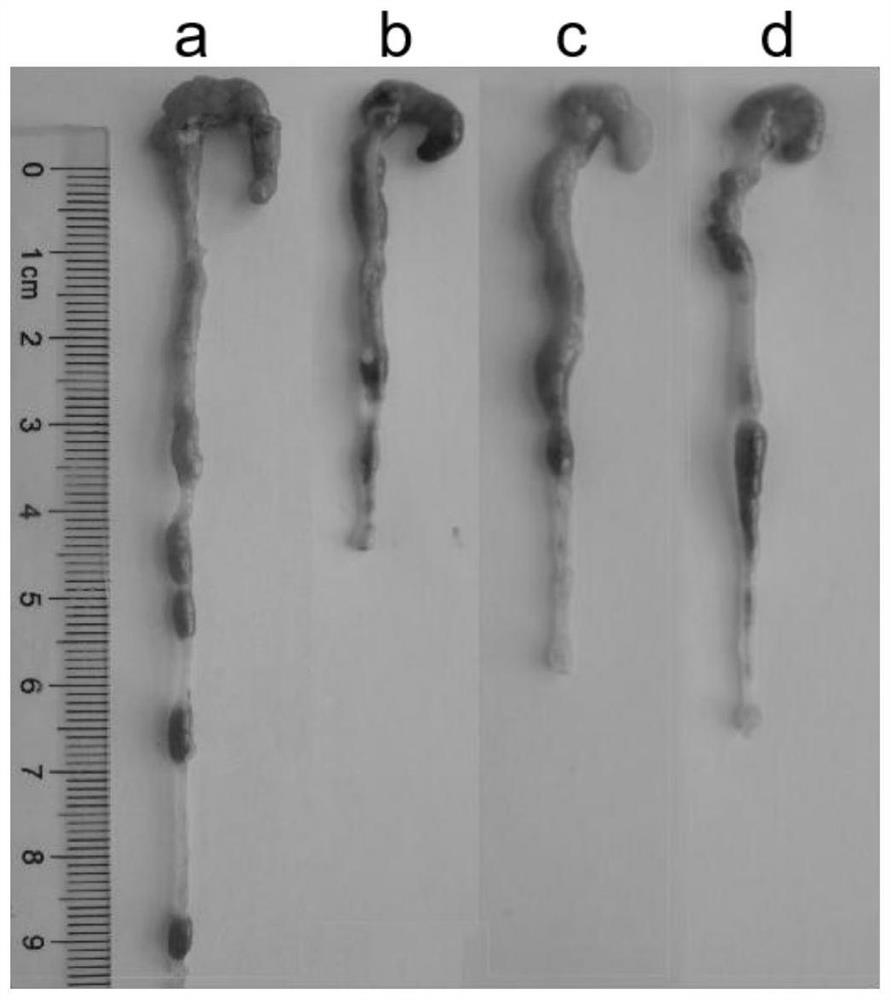
[0034] C57BL / 6J female mice (20±2g) with uniform body weight were selected, and the internationally accepted ulcerative colitis modeling method was adopted (Gastroenterology 2002,123:256...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com