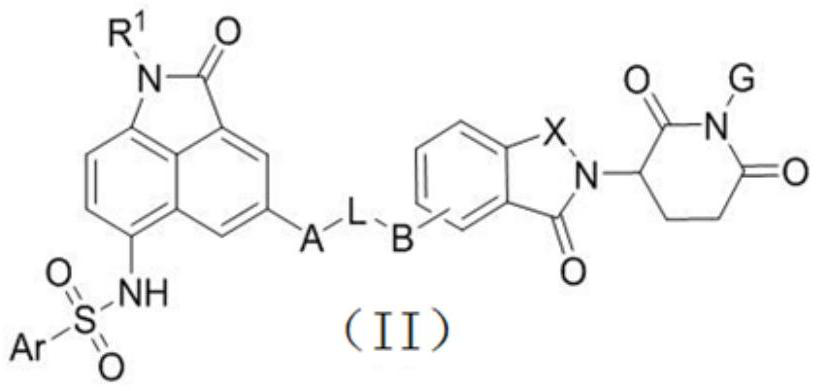
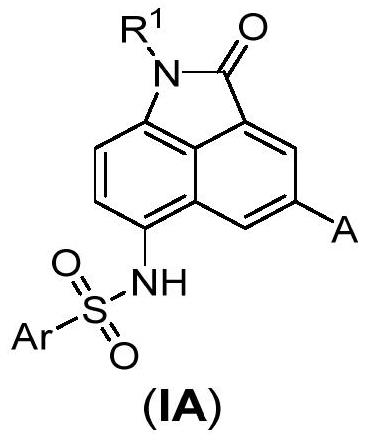
Benzoindole bifunctional molecular derivatives as well as preparation method and application thereof
A bifunctional molecule, benzindole technology, applied in the bifunctional molecular derivatives of benzindole and its preparation, as a BET protein inhibitor degradation agent, can solve the problem of poor patient tolerance, single structure, compound PK properties and poor in vitro properties, etc., to achieve high-efficiency induction of apoptosis, good protein affinity and selectivity, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
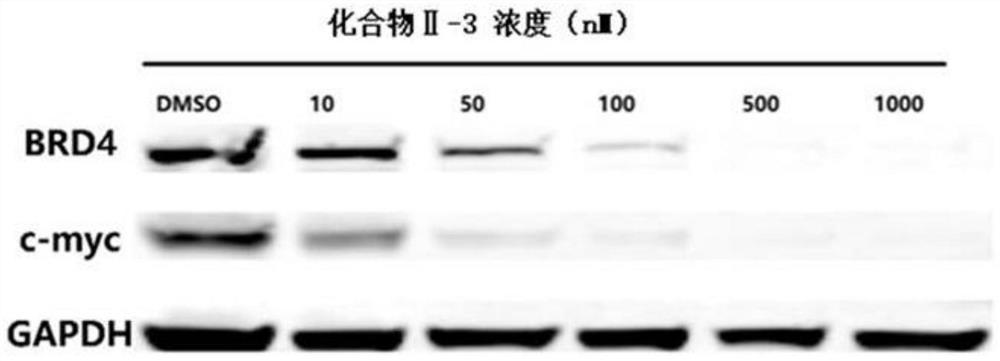
Image
Examples
Embodiment 1
[0103] 3-(6-((2-methoxyphenyl)sulfonylamino)-1-methyl-2-oxo-1,2-dihydrobenzo[cd]indol-4-yl)propanoic acid (I-1g)
[0104]
[0105] Step 1: 5-Bromo-1H,3H-benzo[de]isochromene-1,3-dione (I-1a)
[0106] Add 1,8-naphthalene dicarboxylic anhydride (10.0g, 50.5mmol) and silver sulfate (7.9g, 25.25mmol, 0.50equiv) into concentrated sulfuric acid (200mL) solution, stir at room temperature for 30 minutes, then add liquid bromine ( 3.2mL, 63.0mmol, 1.26equiv), heated to 60°C, heated for 8-10 hours, then cooled to 20°C. The completion of the reaction was checked by TLC, and the silver bromide by-product solid was filtered off to obtain a clear orange solution. The orange solution was added dropwise to an ice-water mixture (1 L), stirred at room temperature for 20 min, and an off-white solid was obtained by filtration. The filter cake was washed with water (50mL), then washed with cold ethanol (100mL×2), and dried in a constant temperature vacuum oven at 60°C to obtain a white solid ...
Embodiment 2
[0120] N-(3-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindol-4-yl)amino)ethyl)-3-(6 -((2-Methoxyphenyl)sulfonylamino)-1-methyl-2-oxo-1,2-dihydrobenzo[cd]indol-4-yl)propionamide (Ⅱ-1 )
[0121]
[0122] Step 1: 2-(2,6-dioxopiperidin-3-yl)-4-fluoroisoindole-1,3-dione (Ⅱ-1a)
[0123] Weigh 4-fluoroisobenzofuran-1,3-dione (2g, 12.04mmol, 1equiv) into an eggplant-shaped bottle, dissolve it with 20mL AcOH, add 3-aminopiperidine-2,6-dione hydrochloride Salt (2.18 g, 13.24 mmol, 1.1 equiv) and AcOK (2.36 g, 24.08 mmol, 2 equiv). Stir and heat at 60°C for 12 hours. TLC detected that the reaction was complete, extracted with EA, washed the organic layer with saturated brine, Mg 2 SO 4 After drying, the solvent was distilled off under reduced pressure, and purified by silica gel column chromatography to obtain a white solid (II-1a) (2.13 g, 7.71 mmol), yield: 64%. MS m / s(ESI)[M+H]+ : 277.1.
[0124] Step 2: tert-butyl (2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindol-4-yl)amino...
Embodiment 3
[0131] N-(3-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindol-4-yl)amino)propyl)-3-(6 -((2-methoxyphenyl)sulfonylamino)-1-methyl-2-oxo-1,2-dihydrobenzo[cd]indol-4-yl)propionamide (Ⅱ-2 )
[0132]
[0133] Step 1: tert-Butyl (3-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindol-4-yl)amino)propyl)carbamate Esters (Ⅱ-2a)
[0134] Its preparation method is the same as Step 2 of Example 2: (2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindol-4-yl)amino) The preparation of ethyl) tert-butyl carbamate (Ⅱ-1b) is similar to 2-(2,6-dioxopiperidin-3-yl)-4-fluoroisoindole-1,3-dione ( II-1a) was used as the raw material, and the dosage was 300mg (1.09mmol). Finally, a light yellow solid (II-2a) (182mg, 0.42mmol) was obtained, with a yield of 39%.
[0135] Step 2: 4-((3-aminopropyl)amino)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione (Ⅱ-2b) .
[0136] Step 3: N-(3-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindol-4-yl)amino)propyl)-3 -(6-((2-methoxyphenyl)sulfonylamino)-1-methyl-2-oxo-1,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com