Preparation method of furandicarboxylic acid diamine polymer
A technology of furandicarboxylic acid diamine and furandicarboxylic acid is applied in the field of preparation of furandiamine diamine high polymer, can solve the problems of complicated and expensive processing process, and achieves the effects of easy control, green preparation process and improved molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
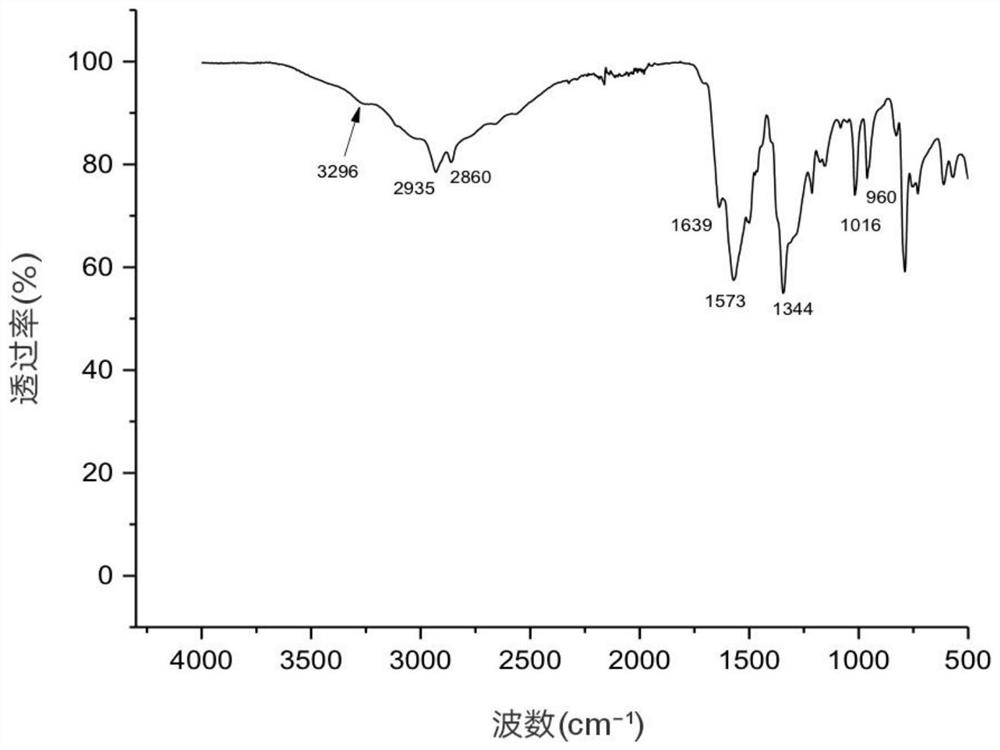
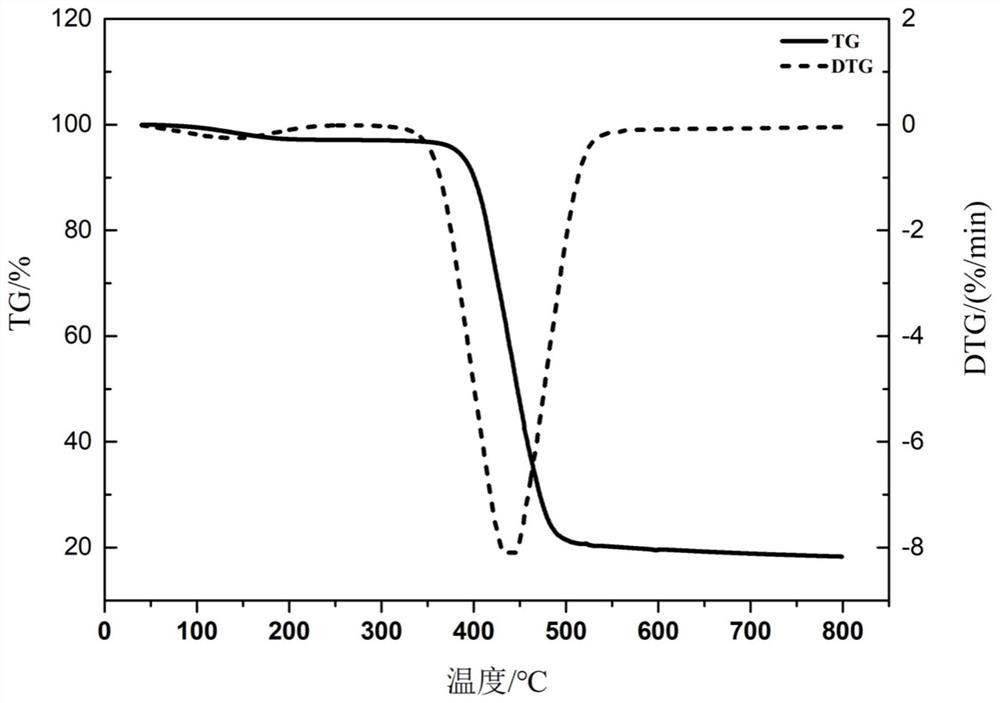
Image
Examples
preparation example Construction
[0019] The invention provides a kind of preparation method (abbreviation method) of furandiamine high polymer, it is characterized in that the method comprises the following steps:
[0020] (1) Dissolve at least one of diamine and furandicarboxylic acid, furandicarboxylic acid chloride compound, furandicarboxylic acid bromide compound or furandicarboxylic ester compound in DMF, absolute ethanol and other solvents respectively, and then mutually After mixing (preferably pour the diamine solution into furandicarboxylic acid, furandicarboxylic acid chloride compound, furandicarboxylic acid bromide compound or furandicarboxylic acid ester solution), under the protection of inert gas, react to form a precipitate, After the precipitate no longer increases, the precipitate is cooled, filtered, and vacuum-dried to obtain the compound salt;
[0021] (2) Add the compound salt into the reactor, and continue vacuuming after introducing the inert gas. Under the catalysis of the catalyst, f...
Embodiment 1
[0036] (1) Dissolve 2,5-furandicarboxylic acid and 1,6-hexanediamine in absolute ethanol at a molar ratio of 1:1, then slowly pour the 1,6-hexanediamine solution into 2,5 - In the furandicarboxylic acid solution, under the conditions of nitrogen protection and magnetic stirring, the reaction temperature is 50° C., and the water bath reacts for 12 hours to produce a precipitate. The precipitate is cooled, filtered, and vacuum-dried for 12 hours to obtain a compound salt; the obtained compound salt is in the form of a white powder.
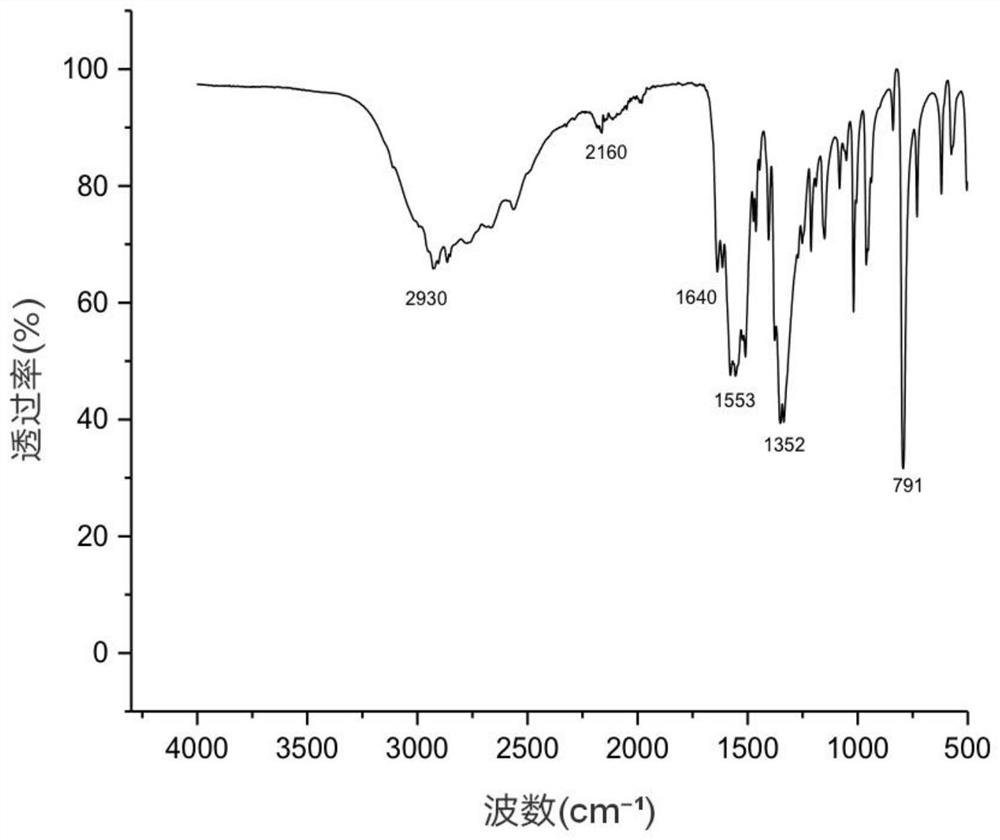
[0037] The composite salt obtained was characterized by infrared spectroscopy test, figure 1 It is the infrared spectrum of compound salt, 2930cm -1 at methylene (-CH 2 -) stretching vibration peak, 1640cm -1 is the carboxylate ion (COO -) and amino ions (NH 3 + ) combined characteristic peak, 1553cm -1 At the peak of amide II, 791cm -1 The position is the in-plane bending vibration absorption peak of =C-H on the furan ring, indicating that t...
Embodiment 2
[0047] The specific reaction conditions are the same as in Example 1, except that the molar ratio of 2,5-furandicarboxylic acid to 1,6-hexanediamine is changed to 1:0.8.
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com