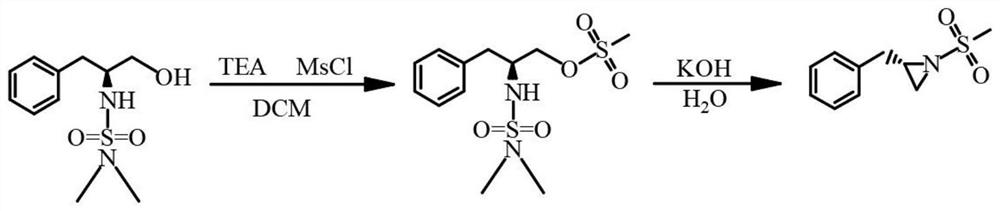
One-pot production process of chiral sulfonyl cyclopropylamine derivative
A technology of sulfonylcyclopropylamine and sulfonylcyclopropylamine, which is applied in the field of one-pot production process of chiral sulfonylcyclopropylamine derivatives, can solve the problems of large amount of three wastes, low total yield and high production cost, and achieve The effect of reducing the amount of three wastes produced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 (comparative example)
[0030] Add L-phenylpropanol (10.0g, 0.066mol, 1.0eq) to the reaction flask, then add dichloromethane (133g), cool to 0°C, add DIPEA (21.4g, 0.253mol, 2.5eq) dropwise into the reaction flask. After the dropwise addition was completed, dimethylaminosulfonyl chloride (10.0 g, 0.066 mol, 1.05 eq) was continued to be added dropwise. After dropping, react overnight at room temperature. The reaction solution was washed successively with saturated aqueous ammonium chloride solution (50.0 g), 1N hydrochloric acid (50.0 g), and saturated brine (50.0 g×2). Anhydrous sodium sulfate (10.0 g) was dried, filtered, and the filtrate was concentrated under reduced pressure in dichloromethane to dryness to obtain 13.2 g of yellow oil, yield 77.2%, HPLC: 82%.
[0031] Add raw materials (13.2g, 0.051mol, 1.0eq) and dichloromethane (133.0g) to the reaction flask, cool down to 0°C, and add triethylamine (5.7g, 0.056mol, 1.1eq) dropwise. After the dropp...
Embodiment 2
[0034] The reaction system was dried, and L-phenylpropanol (500 g, 3.31 mol, 1.0 eq) and DCM (2500 mL) were added, and nitrogen half-replaced 5 times. Stir and heat up to 35°C, the reaction solution is clear. Triethylamine (419 g, 4.14 mol, 1.25 eq) was added under nitrogen. Control the temperature at 35-40°C, and add dimethylaminosulfonyl chloride (570g, 3.97mol, 1.2eq) dropwise. After 25 minutes of dropwise addition, keep the internal temperature at 35-40°C and stir for 2 hours, and proceed directly to the next step.
[0035] The reaction solution was cooled to 16°C (a large amount of solid precipitated, which should be the hydrochloride of triethylamine), and triethylamine (368g, 3.64mol, 1.1eq) was added. The temperature was controlled below 35°C and methanesulfonyl chloride (400g , 3.49mol, 1.05eq). After dropping, stir naturally for 30min, gradually add ammonium chloride (221g, 4.13mol, 1.25eq) in water (679g) solution (total 900g) in the reaction solution, until the ...
Embodiment 3
[0038] The reaction system was dried, and L-phenylpropanol (75.0 g, 0.496 mol, 1.0 eq), DCM (250 mL) was added, and nitrogen half-replaced 5 times. Stir and heat up to 35°C, the reaction solution is clear. Triethylamine (62.8 g, 0.621 mol, 1.25 eq) was added under nitrogen protection. Control the temperature at 35-40°C, and add dimethylaminosulfonyl chloride (85.5g, 0.596mol, 1.2eq) dropwise. The dropwise addition was completed in 20 minutes. (When dropping to about 50%, it starts to release heat, adjust the temperature of the external bath water in time to keep the internal temperature between 35-40°C. After dropping, keep the internal temperature at 35-40°C and stir for 4h. Cool down and stir overnight (13h) , proceed directly to the next step.
[0039] Triethylamine (55.2 g, 0.546 mol, 1.1 eq) was added to the reaction solution, and methanesulfonyl chloride (60.0 g, 0.527 mol, 1.05 eq) was added dropwise at a temperature controlled below 25-35°C. 35min dripping complete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com