Calixarene compound, curable composition and cured product
A technology of calixarene and compound, applied in the field of calixarene compound, can solve the problem of insufficient performance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0268] The following production examples and examples are given to describe the present invention more specifically, but the present invention is not limited to these examples. Parts and % in the examples are all based on mass unless otherwise specified.
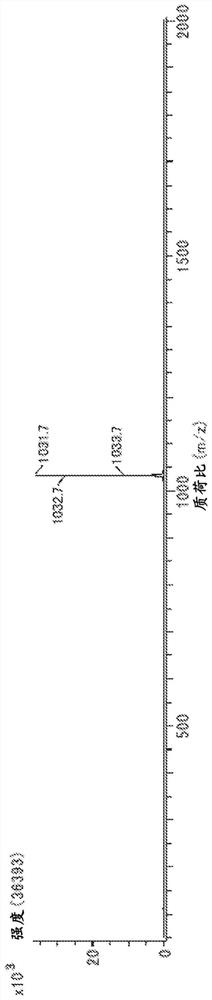
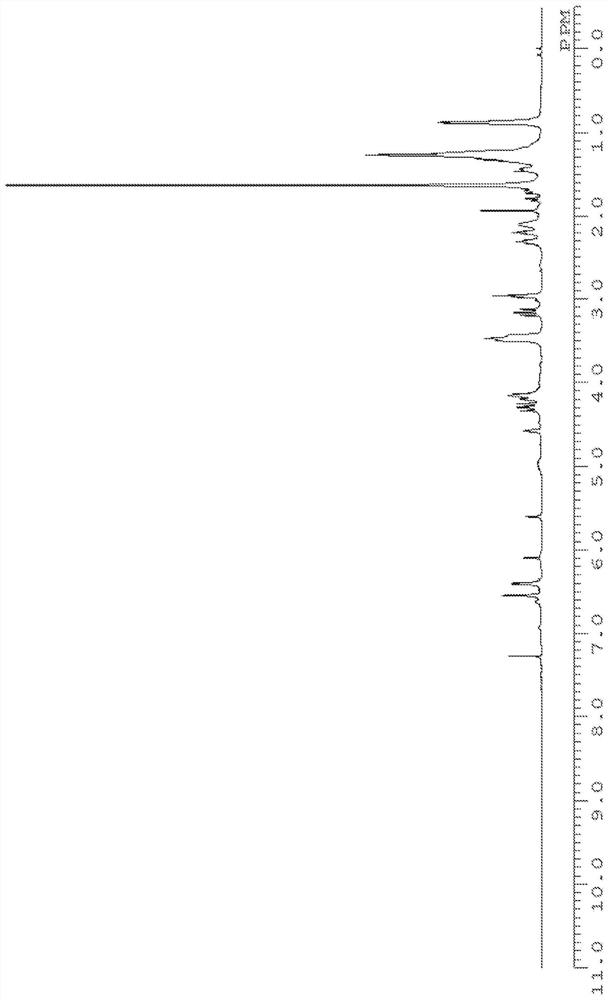
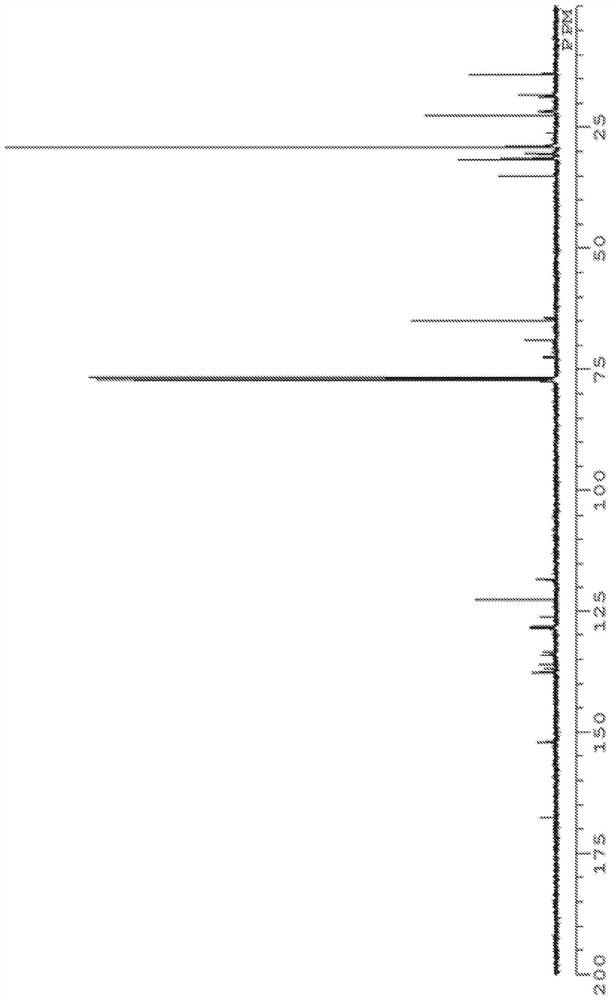
[0269] The structure of product (calixarene compound) is determined by measuring according to the following conditions 1 H-NMR, 13 C-NMR, FD-MS performed.
[0270] 1 H-NMR was measured under the following conditions using "JNM-ECM400S" manufactured by JEOL RESONANCE.
[0271] Magnetic field strength: 400MHz
[0272] Number of points: 16 times
[0273] Solvent: deuterated chloroform
[0274] Sample concentration: 2mg / 0.5ml
[0275] 13 C-NMR was measured under the following conditions using "JNM-ECM400S" manufactured by JEOL RESONANCE.
[0276] Magnetic field strength: 100MHz
[0277] Number of points: 1000 times
[0278] Solvent: deuterated chloroform
[0279] Sample concentration: 2mg / ...
Synthetic example 1
[0288] In a 20L separate four-neck flask equipped with a stirring device, a thermometer and a reflux cooling tube, quickly add tert-butyl calix[4]arene 1000g (1.54mol), phenol 1159g (12.32mol) and dehydrated toluene 9375ml, under nitrogen Flow down and stir at 300 rpm. The raw material tert-butylcalix[4]arene was suspended without being dissolved. Next, 1643 g (12.32 mol) of anhydrous aluminum chloride (III) was added in portions while cooling the flask on ice. The solution became a light orange transparent solution, and anhydrous aluminum(III) chloride precipitated at the bottom. After reacting at room temperature for 5 hours, the contents were transferred to a 1 L beaker, and 20 Kg of ice, 10 L of 1N hydrochloric acid, and 20 L of chloroform were added to terminate the reaction. Become a pale yellow transparent solution. The reaction mixture was transferred to a separatory funnel, and the organic layer was separated. Next, the aqueous layer was extracted three times wi...
Synthetic example 2
[0292] Add 205 g (1.52 mol) of n-hexanoyl chloride and 709 g (9.44 mol) of nitroethane into a 2L four-necked flask equipped with a stirring device, a thermometer, and a reflux cooling tube, and stir. Next, while ice-bathing the flask, 243 g (1.82 mol) of anhydrous aluminum chloride (III) was charged in several portions. The solution became a light orange transparent solution. After stirring at room temperature for 30 minutes, the intermediate (α-1) was added in divided portions at 100 g (0.236 mol). The reaction proceeded while foaming, and an orange transparent solution was obtained. After reacting at room temperature for 5 hours, the contents were slowly transferred to a 2 L beaker to which 450 ml of chloroform and 956 g of ice water were added to stop the reaction. Next, after adding 1N hydrochloric acid until the pH reached 1, the reaction mixture was transferred to a separatory funnel, and the organic layer was separated. Next, the aqueous layer was extracted three t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com