Human extracellular matrix protein 1 detection kit
A kit and protein technology, applied in the field of diagnosis, can solve problems such as failure to detect liver fibrosis, failure to reflect the whole, patient death, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0233] Example 1 Obtaining of human ECM1 gene
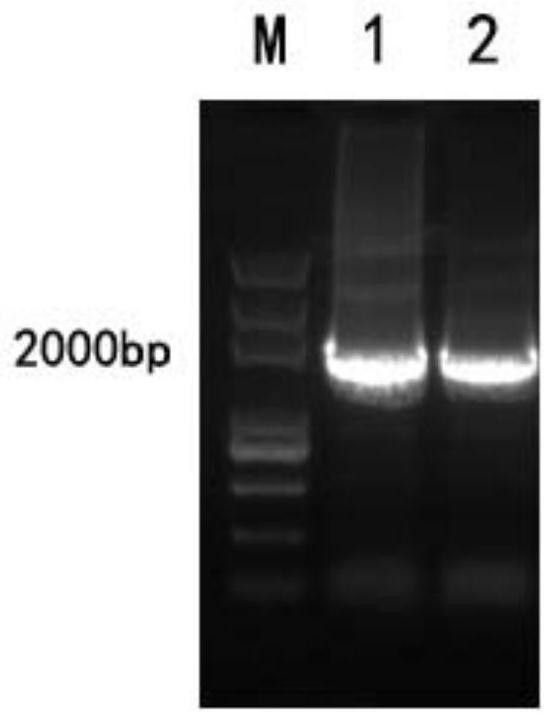
[0234] Using the THp1 cells preserved in our laboratory as a template, a fragment of about 1900bp was amplified with Human-ECM1-sense and Human-ECM1-anti primers, and the PCR product was recovered by running rubber and tapping, and then digested with NotI and BamHI double enzymes, and connected to the same On the pcDNA3.1-FC expression vector treated with double enzyme digestion, transform Escherichia coli DH5α competent, the plasmid insert fragment was verified by bacterial liquid PCR, and the positive clone was selected for DNA sequencing (sequencing was completed by Bosun Biological Co., Ltd.) after the reagents were correct. The plasmid was extracted using the cassette to obtain the expression plasmid pcDNA3.1-Fc-hECM1.
[0235] From figure 1 It can be seen from the figure that the human ECM1 gene was successfully obtained with good purity and content.
Embodiment 2
[0236] The preparation of embodiment 2 monoclonal antibody
[0237] 2.1 Monoclonal Antibody Protein G Affinity Chromatography Purification
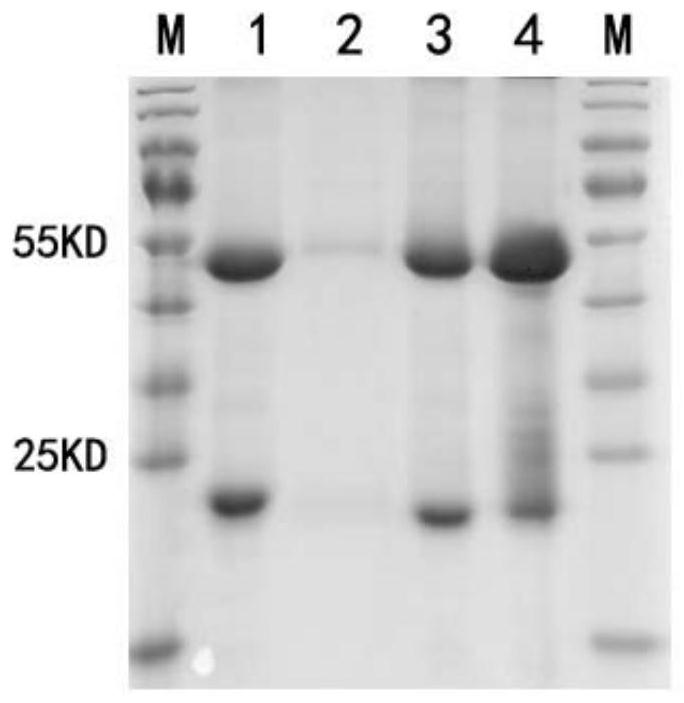
[0238] Inject 0.5 mL pristane intraperitoneally into the mice. After 7-10 days, intraperitoneally inject 0.5mL 1×10 6 hybridoma cells. The growth of the mice was observed every day, and abdominal swelling was visible in about 7 days, and the ascites was collected in time. The ascites fluid collected from 4 mice was preliminarily purified by 50% saturated ammonium sulfate precipitation, and then purified by Protein G-sepHarose4B. The protein not bound to the column was eluted by PB, and the protein was finally eluted by 0.1mol / L pH2.7glycine. After protein G was collected and purified, the antibody was added to the loading buffer and boiled, loaded, electrophoresed by SDS, and stained with Coomassie brilliant blue. Check the effect of antibody purification.
[0239] Experimental results such as figure 2 As shown, the four antibodies...
Embodiment 3
[0242] Example 3 Characterization of Anti-hECM1 monoclonal antibody after purification
[0243] The amount of biotin reagent added was calculated according to the concentration of the purified antibody, and the 4 murine monoclonal antibodies were labeled with Biotin and purified through a desalting column. Then, the affinity of the labeled monoclonal antibody to the standard was detected by indirect ELISA, and the standard was serially diluted (10ng / mL, 5ng / mL, 2.5ng / mL, 1.25ng / mL, 0.625ng / mL, 0.31ng / mL mL, 0.15ng / mL, 0.075ng / mL). The affinities of the Biotin-labeled antibodies to the standards with different concentrations were tested respectively.
[0244] Experimental results such as Figure 4 As shown, the OD value of the purified and Biotin-labeled antibody was much higher than that of the negative control group, indicating that the affinity activity of the purified and labeled antibody was not affected by purification and Biotin labeling, and the No. 4 and No. 3 antibo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com