A kind of dimer prodrug and its preparation method and application
A technology of dimer and prodrug, which is applied in the field of dimer prodrug and its preparation, can solve the problems of observing prodrug activation, etc., and achieve the effect of enhancing treatment, enhancing therapeutic effect and reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
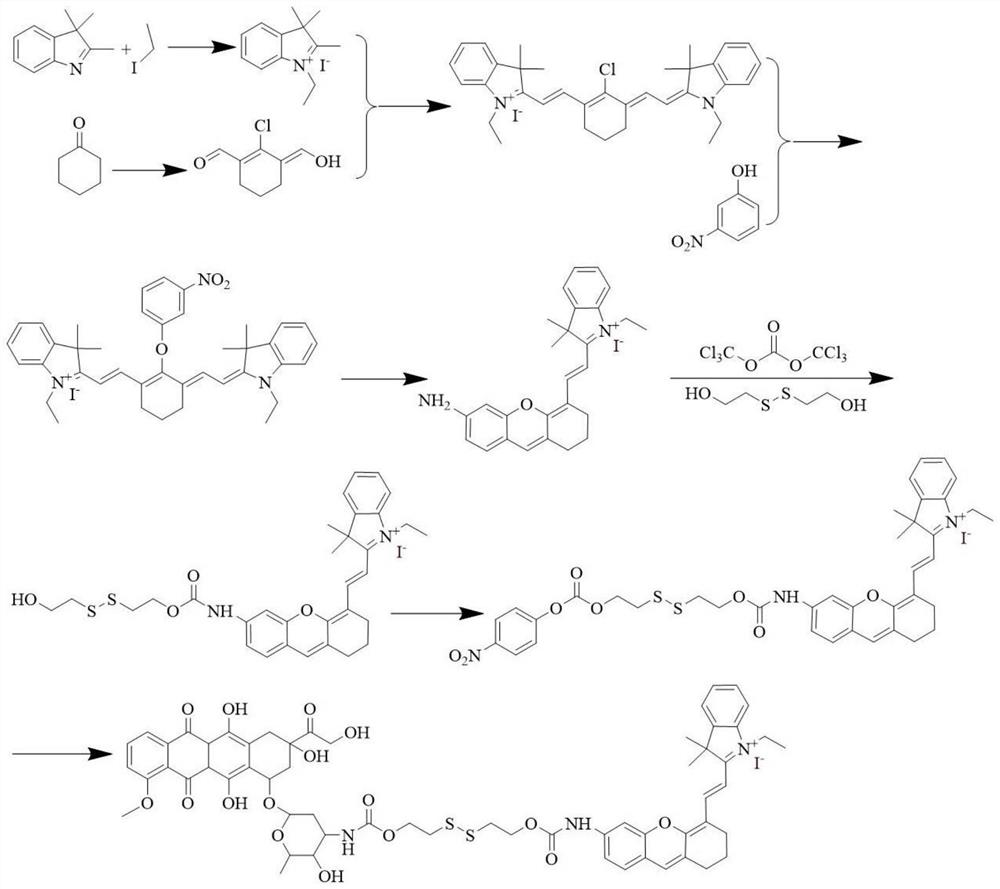
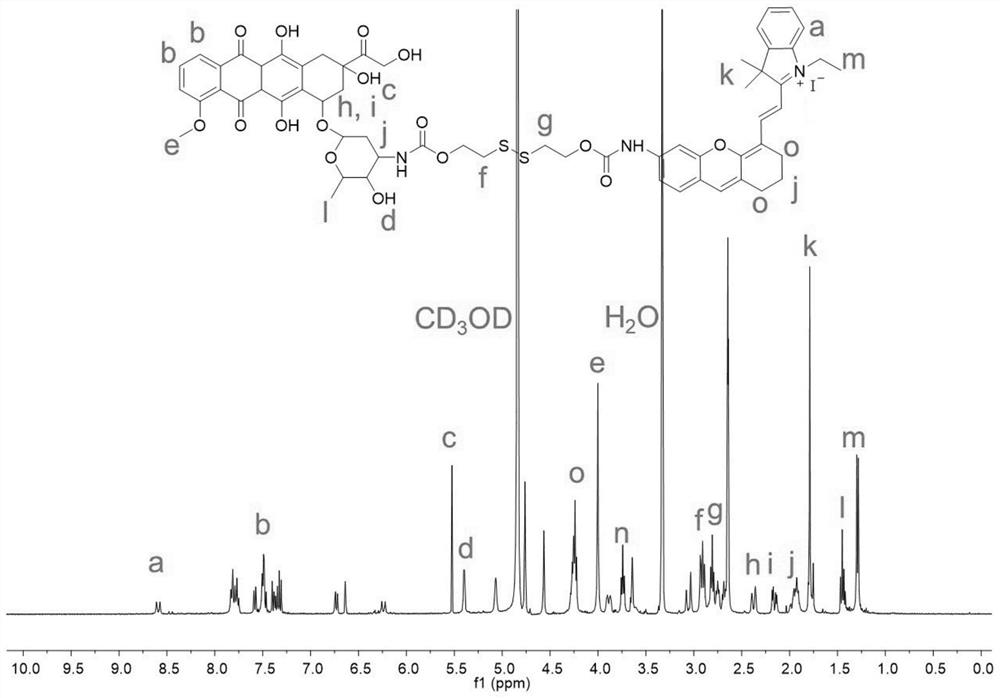
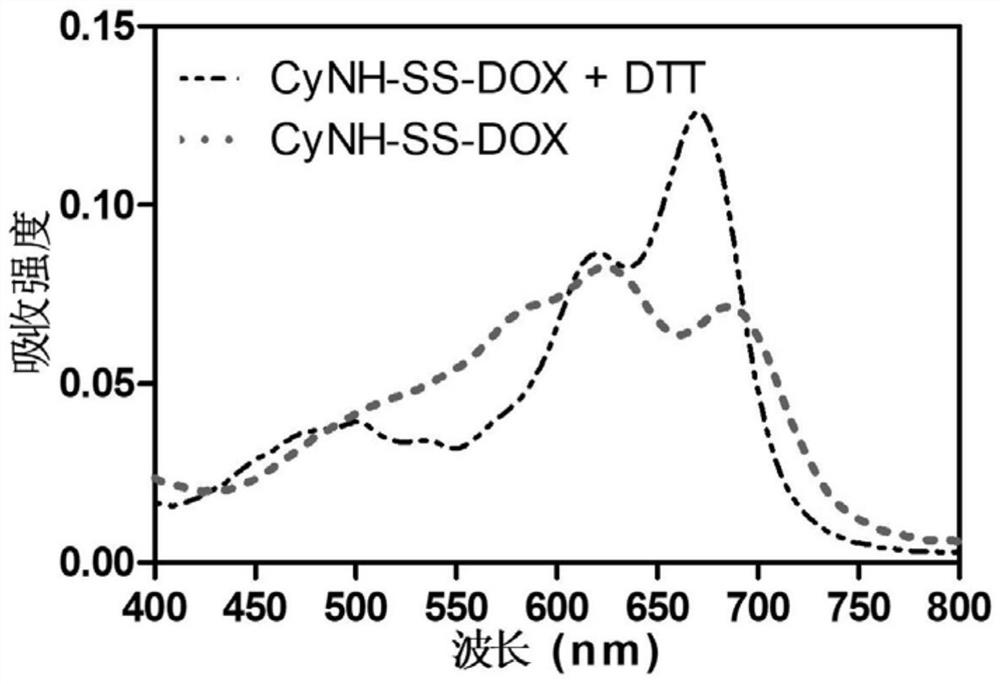
[0059]A dimer prodrug, its preparation method comprises the following steps (synthetic route such as figure 1 shown):
[0060] 1) Disperse 1.44g (9.0mmol) of 2,3,3-trimethylindole and 7.02g (45.0mmol) of iodoethane in 30mL of acetonitrile, heat to reflux for 24h, then remove the solvent under reduced pressure, and use The crude product obtained by washing with n-hexane was obtained 3 times to obtain (white solid);
[0061] 2) Under a nitrogen atmosphere, mix 3mL (40.95mmol) of dimethylformamide and 15mL of dichloromethane in an ice bath, and then add 20mL of a dichloromethane solution of phosphorus oxychloride (containing phosphorus oxychloride 2.63mL (17.25mmol)) was added dropwise, and 0.74g (7.5mmol) of cyclohexanone was added, heated and stirred to reflux for 3h, then the reaction mixture was poured into ice and left to stand for 12h, filtered to obtain (pale yellow solid);
[0062] 3) 1.89g (6.0mmol) of 0.51g (3.0mmol) and 0.5 g (6.0 mmol) of sodium acetate were...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com