Reagent card for quantitatively detecting helicobacter pylori antibody by fluorescence chromatography and detection method
A technology of Helicobacter pylori and fluorescence chromatography, which is applied in biological testing, measuring devices, immunoassays, etc., can solve the problems of difficult research and development, and achieve the effect of reasonable design and quick and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
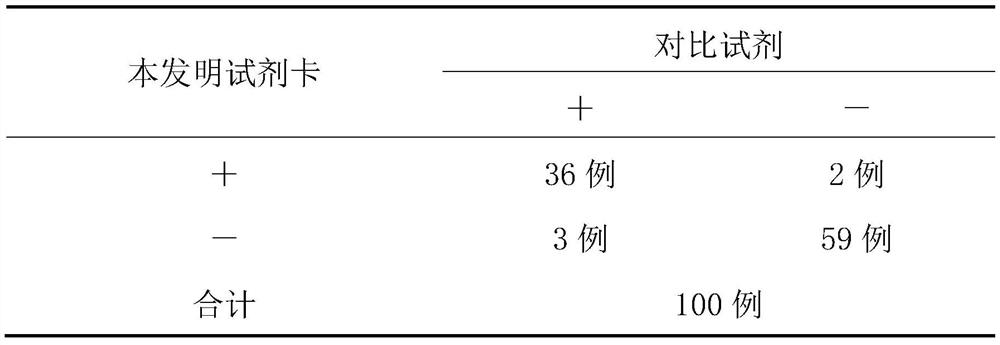
Examples
Embodiment
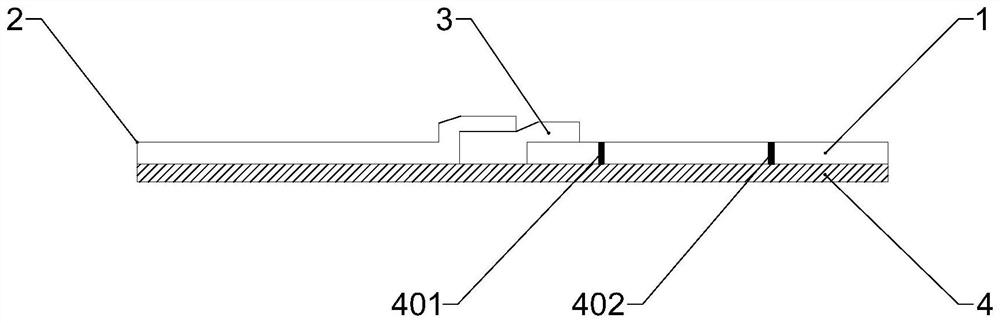
[0024] The reagent card for the quantitative detection of Helicobacter pylori antibody by fluorescence chromatography in this embodiment was prepared according to the following steps.
[0025]1. Helicobacter pylori recombinant protein antigen-coated latex microspheres labeled with lanthanide elements: the particle size of the latex microspheres is 100nm, and the Helicobacter pylori recombinant protein antigen is coated with lanthanide-labeled latex microspheres Ball 6h, coating concentration is 30μg Helicobacter pylori recombinant protein antigen / 1mg latex microsphere labeled with lanthanide, centrifuged under the condition of 14000r / min for 30min, isolate the latex microsphere coated Helicobacter pylori recombinant protein antigen, and washed 3 times with 0.01mol / L PBS, and then added to a final concentration of 1-2% BSA and 1-2% casein to seal the latex microspheres coated with Helicobacter pylori recombinant protein antigen for 30 minutes; Wash the latex microspheres of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com