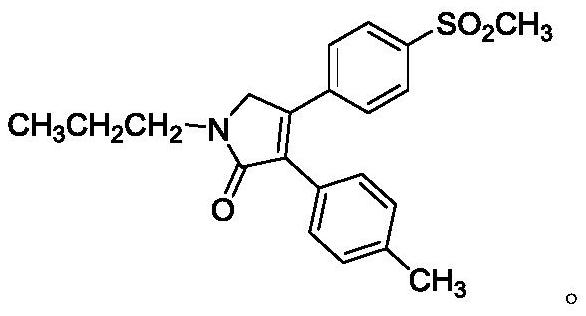
Application of combination of imrecoxib and tramadol in preparation of medicine for treating pain
A technology of tramadol and its use, which is applied in the field of the use of the combination of Erecoxib and tramadol in the preparation of drugs for the treatment of pain, and can solve the problems of increasing the risk of tramadol addiction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
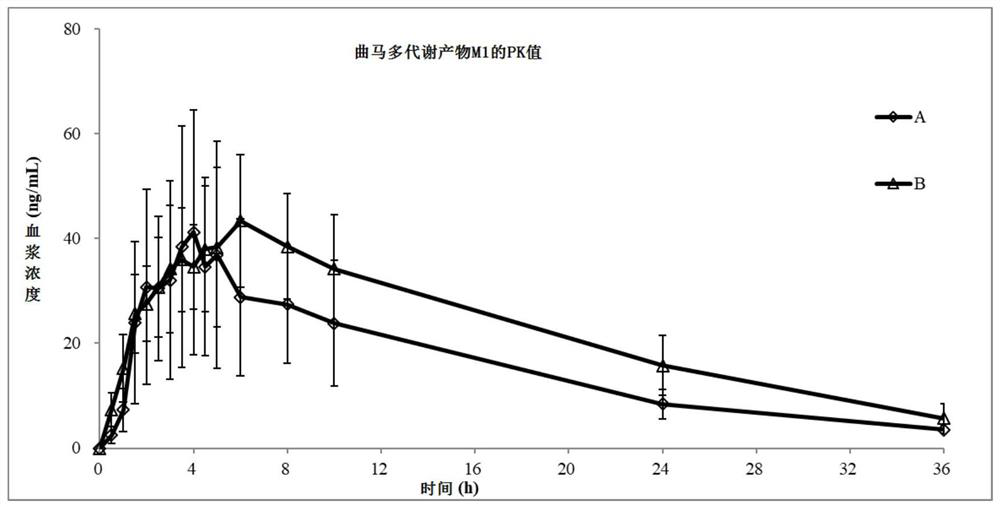
[0075] Example 1. To verify the effect of the combined use of Erecoxib and tramadol on the exposure (AUC) of O-desmethyl tramadol (M1) in healthy subjects compared with single-drug tramadol.
[0076] 1. Experimental drugs
[0077] Shumin (Germany Gruntel Co., Ltd., tramadol hydrochloride sustained-release tablets, specification 100 mg), tramadol hydrochloride, and erecoxib.
[0078] 2. Experimental method
[0079] The subjects were admitted to the clinical trial ward in the afternoon of the day before the test, had a uniform light diet in the evening, and then fasted without water for 10 hours overnight. At around 8:00 the next morning, they were orally administered tramadol hydrochloride 100 mg and Erecoxib 100 mg on an empty stomach. Composition or antiseptic. Except before taking the medicine and 1 hour after taking the medicine, you can drink water as needed at other times, and eat a standard meal 4 hours after the medicine (around 12:00). During hospitalization (36 hou...
Embodiment 2
[0087] Embodiment 2, mouse acetic acid writhing model analgesic efficacy test
[0088] Male ICR mice were used as experimental animals, and the analgesic efficacy test of mouse acetic acid writhing model was carried out. Specifically: 104 male ICR mice qualified for quarantine were randomly divided into blank control group, Erecoxib nanocrystal 5, 10, 15, 30 mg / kg group, and tramadol hydrochloride 2.5, 5, 10, 15 mg / kg group. group, Erecoxib nanocrystal and tramadol hydrochloride combined group 2.5+2.5mg / kg, 5+5mg / kg, 10+10mg / kg, 15+15mg / kg group, 8 rats in each group, administration volume Both are 10ml / kg, and the solvent is 0.5% CMC-Na. After a single intragastric administration for 30 minutes, mice in each group were intraperitoneally injected with 1.5% acetic acid solution 5ml / kg to establish a model. Immediately after modeling, the number of times of writhing in mice within 20 min was recorded, and the inhibition rate was calculated (Table 3).
[0089] The preparation ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com