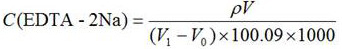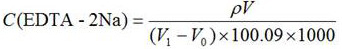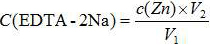Method for calibrating EDTA-2Na (Ethylene Diamine Tetraacetic Acid-2Na) during total hardness measurement
A calibration method and a technology of total hardness, applied in the chemical field, can solve problems such as cumbersome process, time-consuming, increased zinc loss, etc., and achieve the effect of fast method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The known quality control sample concentration is 181±6 mg / L, draw 50.0 mL into a 150 ml Erlenmeyer flask, add a few drops of ammonia to adjust the solution to near neutral, then add 5 ml buffer solution and 5 drops of chrome black T indicator , Immediately titrate with EDTA-2Na standard solution until the solution turns from purple-red to pure blue, and the titer is 9.53 mL. At the same time, a blank test is performed, and the blank EDTA-2Na standard solution is titrated to zero.
[0044] A known =181 mg / L; V=50.0 mL; V 0 =0; V 1 =9.53 mL, substituted into the calculation formula to get =0.0092 mol / L, which is consistent with the calibration result of 0.0091 mol / L using the EDTA-2Na method in GB / T5750.4-2006 in Comparative Example 1, and the relative deviation is 1.09%.
Embodiment 2
[0051] Knowing that the concentration of the total hardness standard solution is 100 mg / L, draw 50.0 mL into a 150 ml Erlenmeyer flask, add a few drops of ammonia to adjust the solution to near neutral, then add 5 ml of buffer solution and 5 drops of chrome black T indicator, Immediately titrate with EDTA-2Na standard solution until the solution turns from purple-red to pure blue, and the titer is 5.42 mL. At the same time, a blank test is performed, and the blank EDTA-2Na standard solution is titrated to zero.
[0052] A known =100 mg / L; V=50.0 mL; V 0 =0; V 1 =5.43 mL, substituting into the calculation formula to get =0.0092 mol / L, which is consistent with the calibration result of 0.0091 mol / L using the EDTA-2Na method in GB / T5750.4-2006 in Comparative Example 2, and the relative deviation is also 1.09%.
Embodiment 3
[0059] The known quality control sample concentration is 181±6 mg / L, draw 50.0 mL into a 150 ml Erlenmeyer flask, add a few drops of ammonia to adjust the solution to nearly neutral, then add 5 ml buffer solution and 5 drops of chrome black T indicator Immediately titrate with EDTA-2Na standard solution until the solution turns from purple-red to pure blue, and the titer is 8.87 mL. At the same time, a blank test is performed, and the blank EDTA-2Na standard solution is titrated to zero.
[0060] A known =181 mg / L; V=50.0 mL; V 0 =0; V 1 =8.87mL, substituting into the calculation formula to get =0.0102mol / L, which is consistent with the calibration result of EDTA-2Na in GB / T5750.4-2006 in Comparative Example 3, which is 0.0100mol / L, and the relative deviation is 2.00%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com