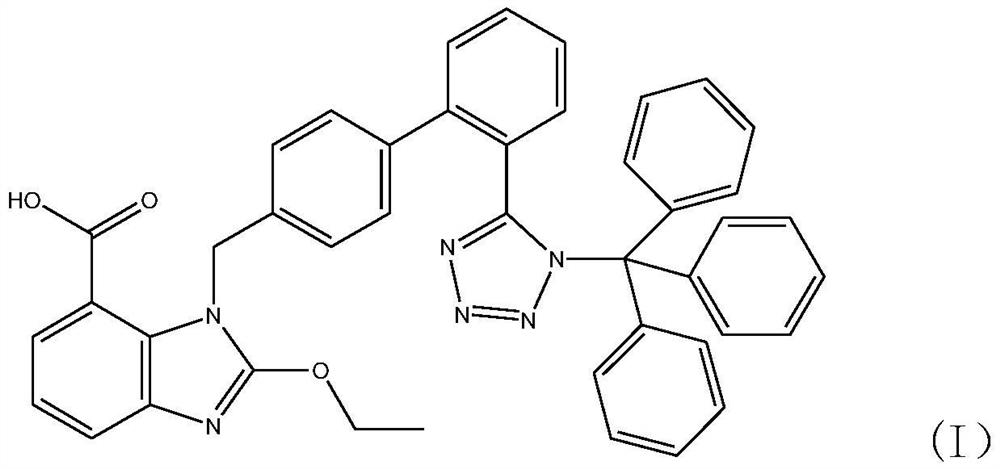
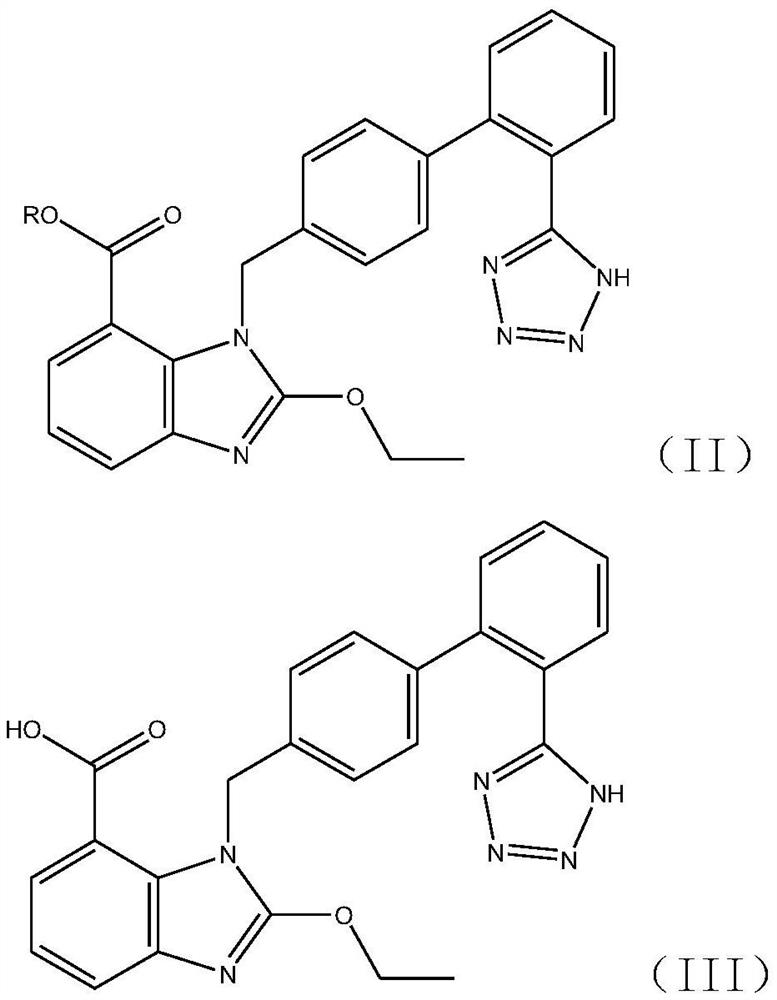
Synthesis method of triphenyl candesartan
A technology for the synthesis of triphenylcandesartan, which is applied in the field of synthesis of triphenylcandesartan, and can solve the problems of long synthetic process flow, loss of raw materials or intermediates, and low yield of triphenylcandesartan , to achieve the effects of improving production efficiency, reducing losses, increasing yield and production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The synthetic method of triphenylcandesartan comprises the following processing steps:
[0054] S1: preparing tributyltin azide, specifically comprising the following steps:
[0055]Step a: Add 200kg of sodium azide into 600kg of water, stir and dissolve to obtain mixed solution a;
[0056] Step b: Control the reaction temperature to 20°C, add 670 kg of tributyltin chloride to the mixed solution a obtained in step a, stir and react for 3.5 hours, and obtain the mixed solution b;
[0057] Step c: add 1200 kg of toluene to the mixed liquid b for stirring and extraction, leave to stand and separate layers, and wash the organic layer with 600 kg of saturated sodium chloride water to obtain a toluene solution of tributyltin azide.
[0058] S2: preparing candesartan intermediate C7, specifically comprising the following steps:
[0059] Add 310kg of ethyl 2-ethoxy-1-[[(2'-cyanobiphenyl-4-substituted)methyl]benzimidazole]-7-carboxylate to the tributyl carboxylate prepared in ...
Embodiment 2
[0073] The synthetic method of triphenylcandesartan comprises the following processing steps:
[0074] S1: preparing tributyltin azide, specifically comprising the following steps:
[0075] Step a: Add 200kg of sodium azide into 600kg of water, stir and dissolve to obtain mixed solution a;
[0076] Step b: Control the reaction temperature to 10°C, add 670kg of tributyltin chloride to the mixed solution a obtained in step a, stir and react for 4 hours, and obtain the mixed solution b;
[0077] Step c: add 1200 kg of toluene to the mixed liquid b for stirring and extraction, leave to stand and separate layers, and wash the organic layer with 600 kg of saturated sodium chloride water to obtain a toluene solution of tributyltin azide.
[0078] S2: preparing candesartan intermediate C7, specifically comprising the following steps:
[0079] Add 310kg of ethyl 2-ethoxy-1-[[(2'-cyanobiphenyl-4-substituted)methyl]benzimidazole]-7-carboxylate to the tributyl carboxylate prepared in st...
Embodiment 3
[0093] The synthetic method of triphenylcandesartan comprises the following processing steps:
[0094] S1: preparing tributyltin azide, specifically comprising the following steps:
[0095] Step a: Add 200kg of sodium azide into 600kg of water, stir and dissolve to obtain mixed solution a;
[0096] Step b: Control the reaction temperature to 25°C, add 670 kg of tributyltin chloride to the mixed solution a obtained in step a, stir and react for 3 hours, and obtain the mixed solution b;
[0097] Step c: add 1200 kg of toluene to the mixed liquid b for stirring and extraction, leave to stand and separate layers, and wash the organic layer with 600 kg of saturated sodium chloride water to obtain a toluene solution of tributyltin azide.
[0098] S2: preparing candesartan intermediate C7, specifically comprising the following steps:
[0099] Add 310kg of ethyl 2-ethoxy-1-[[(2'-cyanobiphenyl-4-substituted)methyl]benzimidazole]-7-carboxylate to the tributyl carboxylate prepared in s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com