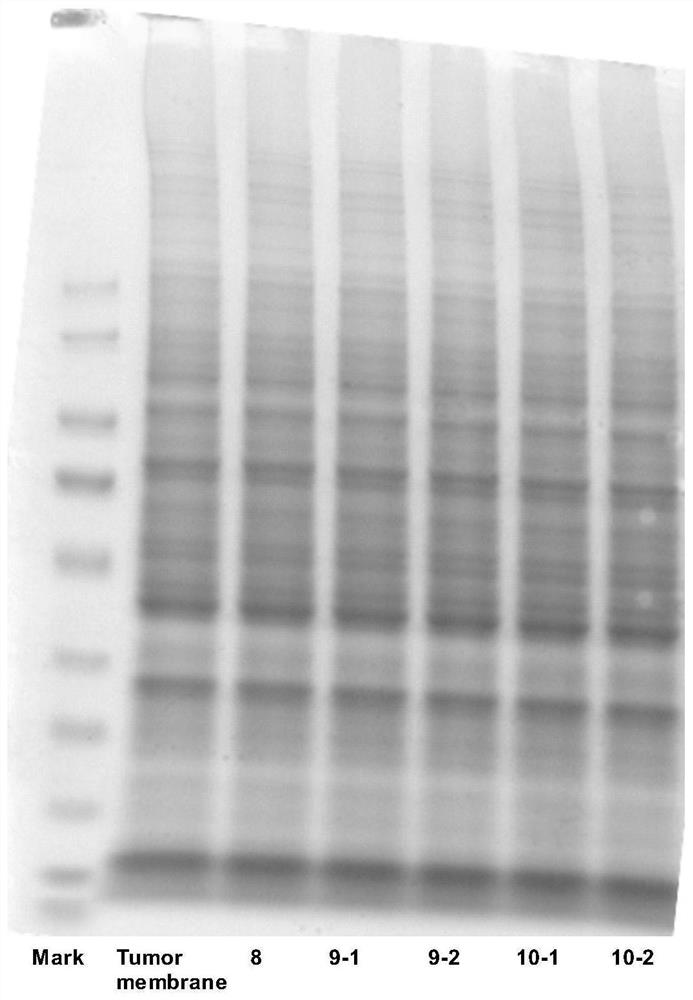
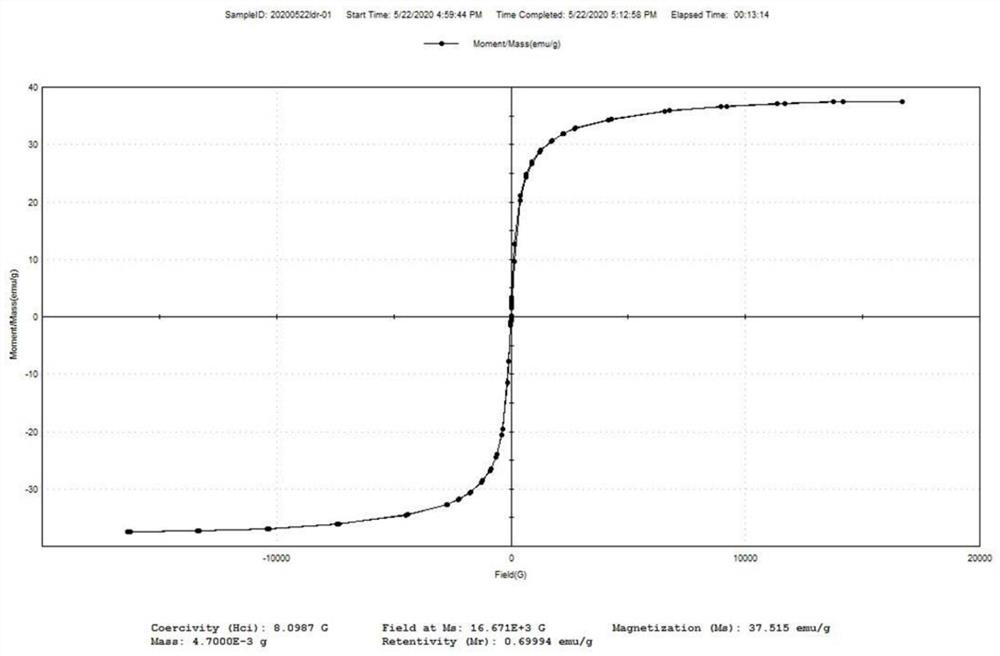
Magnetic targeting cell membrane modified ligand, drug-loading material, preparation method of magnetic targeting cell membrane modified ligand and drug-loading material and application of drug-loading material
A magnetic targeting and cell membrane technology, applied in pharmaceutical formulations, organic chemical methods, chemical instruments and methods, etc., can solve problems such as drug loading rate circulatory system stability defects, and achieve improved stability, drug loading rate, and stability Good, high cell uptake effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of intermediate 2
[0055] Compound 1 (6.0g, 14.1mmol) was dissolved in DCM / DMF (100 / 6mL), then DCC (3.5g, 16.8mmol) was added and stirred for 15min to obtain a mixed solution; then propargylamine (0.9g, 16.8mmol) Dissolve in 25mL DCM, then slowly drop into the above mixture, react at room temperature for 1h. After the reaction was complete, 5.9 g of a white solid (compound 2) was obtained by beating and suction filtration, with a yield of 92%. The intermediate was directly put into the next reaction without purification.
Embodiment 2
[0057] Preparation of intermediate 3
[0058] Dissolve the compound 2 (3.1g, 6.7mmol) prepared in the previous step in DMF (30mL), add piperidine (574.6mg, 6.7mmol) and react at room temperature for 0.5h, point the plate to observe the reaction progress, after the reaction is complete, add EA (ethyl acetate) (300mL) was washed three times with saturated sodium chloride solution, and the EA layer was dried with anhydrous sodium sulfate and concentrated column chromatography (DCM:MeOH=15:1) to obtain a light yellow solid (compound 3) 1.5 g, yield 73%.
[0059] 1 H NMR(600MHz,DMSO)δ8.32(s,1H),6.84(d,J=7.9Hz,2H),3.85(s,2H),3.11(s,1H),2.68(t,J=6.1Hz ,2H),2.50(m,2H),1.58(s,1H),1.38(s,9H).
[0060] ESI-MS:242.1[M+H] + .
Embodiment 3
[0062] Preparation of Intermediate 5
[0063] 4-(diethylamino) salicylaldehyde (5.0g, 25.9mmol) was dissolved in EtOH (100mL), then diethyl malonate (5.5g, 34.5mmol) was dissolved in 50mL EtOH, and slowly dropped into In the EtOH solution of the above-mentioned 4-(diethylamino)salicylaldehyde. Add piperidine (1.8g, 20.7mmol) and react at 90°C for 2h. After the reaction was complete, EtOH was removed under reduced pressure to obtain a mixture containing compound 4. The above mixture was hydrolyzed by adding NaOH solution, finally acidified with citric acid and extracted with ethyl acetate, and the ethyl acetate layer was spin-dried to obtain 6.0 g of a yellow solid (Compound 5), with a yield of 89%.
[0064] 1 H NMR(600MHz,DMSO)δ12.52(s,1H),8.59(s,1H),7.64(d,J=6.0Hz,1H),6.79(dd,J=9.0,1.6Hz,1H),6.57 (s,1H),3.48(q,J=7.0Hz,4H),1.13(t,J=7.0Hz,6H).
[0065] ESI-MS:260.3[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com