Synthetic method of ammonium acetate-mediated benzothiazole compound
A synthetic method and benzothiazole technology, which is applied in the field of synthesis of benzothiazole compounds, can solve the problems of harsh reaction conditions and unstable reaction substrates, and achieve fewer reaction steps, high functional group tolerance, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
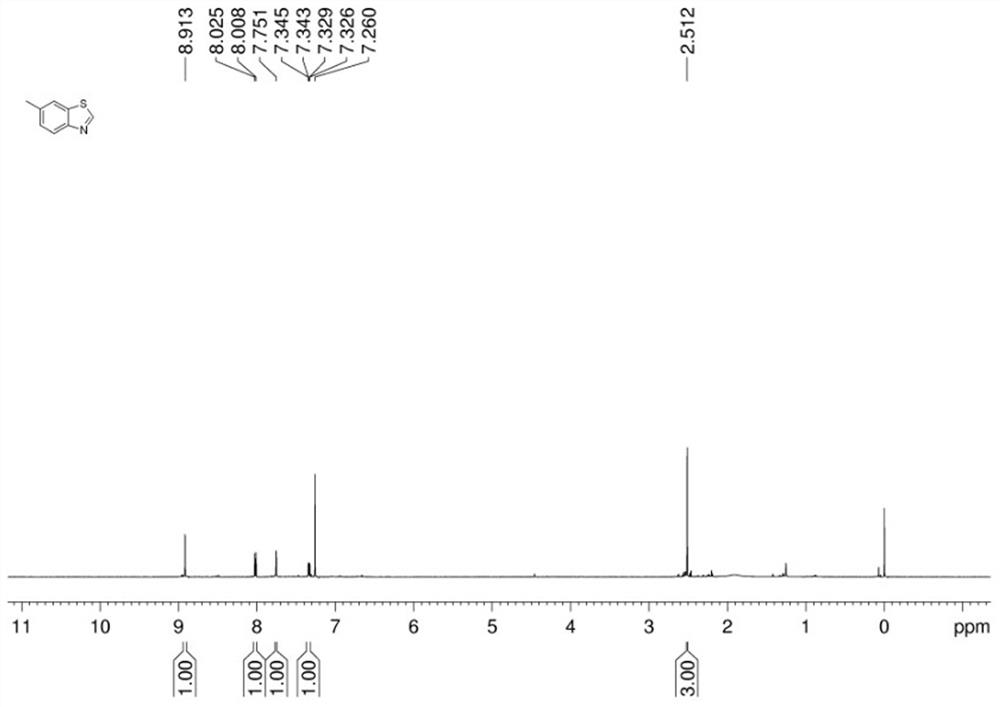
[0042]In a dry Schlenk reaction tube, add 2-iodo-4-methylaniline (0.2 mmol), potassium sulfide (0.6 mmol), dimethyl sulfoxide (2 mL), cuprous iodide (0.04 mmol), acetic acid Ammonium (1.2 mmol), water (80 ul), after adding the sample, vacuumize with an oil pump and inject nitrogen for gas replacement. After three replacements, stop the reaction at 140 °C for 10 hours, and cool to room temperature. The reaction was detected by thin-layer chromatography (TLC). After the reaction of the raw materials was completed, the reaction was terminated, and the mixed solution in the reaction tube was cooled to room temperature. Preliminary treatment of the mixture: pass through a short column, extract, collect the organic layer, spin the powder, and perform column chromatography to obtain the target product with a yield of 95%.
[0043] The hydrogen spectrogram and carbon spectrogram of gained target product are respectively as follows figure 1 and figure 2 As shown, the NMR data are a...
Embodiment 2
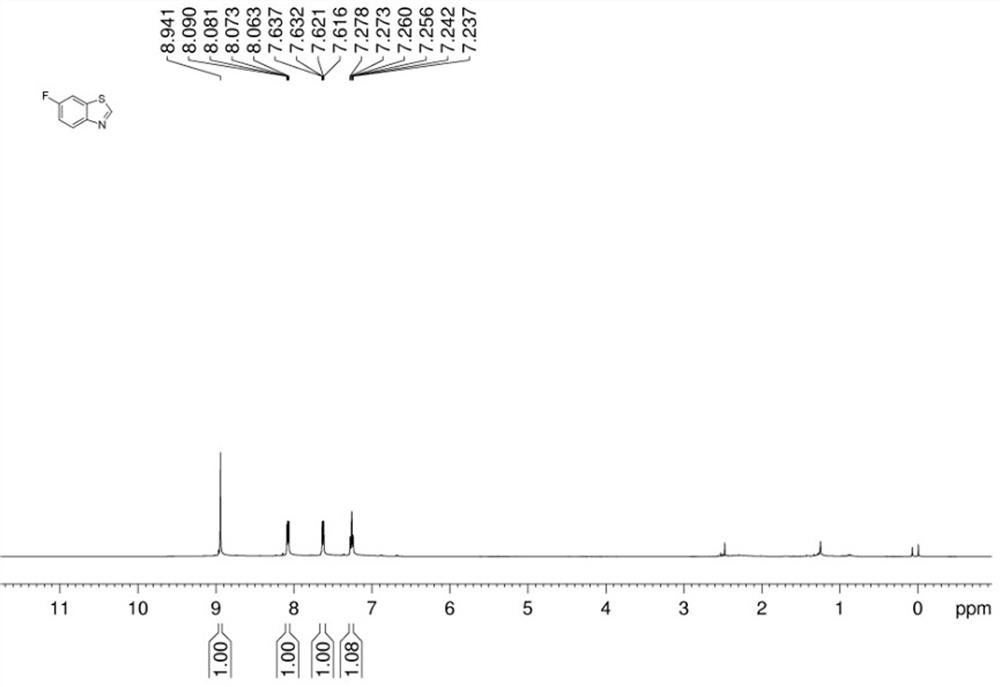
[0048] In a dry Schlenk reaction tube, add 2-iodo-4-methoxyaniline (0.2 mmol), potassium sulfide (0.6 mmol), dimethyl sulfoxide (2 mL), cuprous iodide (0.04 mmol), Ammonium acetate (1.2 mmol), water (80 ul), after adding the sample, vacuumize with an oil pump and inject nitrogen for gas replacement. After three replacements, stop the reaction at 140 °C for 11 hours, and cool to room temperature. The reaction was detected by thin-layer chromatography (TLC). After the reaction of the raw materials was completed, the reaction was terminated, and the mixed solution in the reaction tube was cooled to room temperature. Preliminary treatment of the mixture: pass through a short column, extract, collect the organic layer, spin the powder, and perform column chromatography to obtain the target product with a yield of 50%.
[0049] The hydrogen spectrogram and carbon spectrogram of gained target product are respectively as follows image 3 and Figure 4 As shown, the NMR data are as f...
Embodiment 3
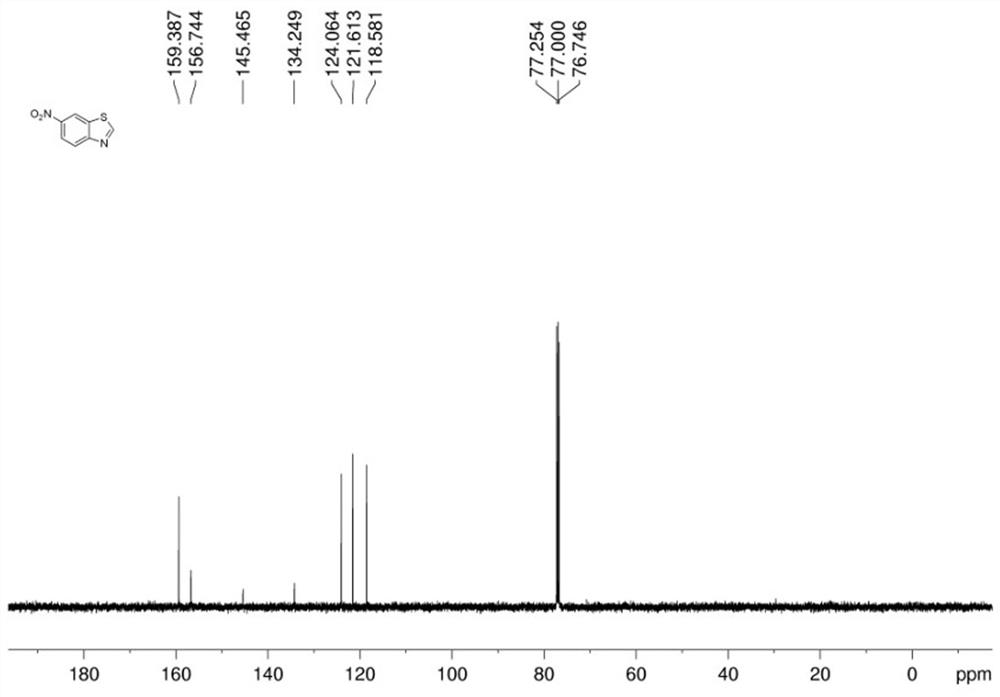
[0054] In a dry Schlenk reaction tube, add 2-iodo-4-cyanoaniline (0.2 mmol), potassium sulfide (0.6 mmol), dimethyl sulfoxide (2 mL), cuprous iodide (0.04 mmol), acetic acid Ammonium (1.2 mmol), water (80 ul), after adding the sample, vacuumize with an oil pump and inject nitrogen for gas replacement. After three replacements, stop the reaction at 140 °C for 11 hours, and cool to room temperature. The reaction was detected by thin-layer chromatography (TLC). After the reaction of the raw materials was completed, the reaction was terminated, and the mixed solution in the reaction tube was cooled to room temperature. Preliminary treatment of the mixture: pass through a short column, extract, collect the organic layer, spin the powder, and perform column chromatography to obtain the target product with a yield of 95%.
[0055] The hydrogen spectrogram and carbon spectrogram of gained target product are respectively as follows Figure 5 and Figure 6 As shown, the NMR data are a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com