Catalyst precursor for hydrocracking reaction and method for hydrocracking heavy oil by using same
一种氢化裂解、催化剂的技术,应用在化学仪器和方法、催化剂活化/制备、金属/金属氧化物/金属氢氧化物催化剂等方向,能够解决生产多等问题,达到降低收率、提高收率、工序容易的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
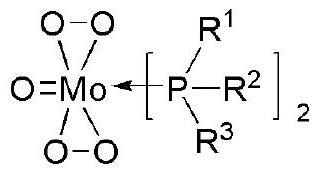
[0119] Using Mo(O)(O 2 ) 2 (PPh 3 ) 2 Hydrocracking reaction of
[0120] Step 1. Add 1 g (6.94 mmol) of MoO to a 100 mL Schlenk tube replaced with argon 3 and hydrogen peroxide (H 2 o 2 , 27%) 2ml, stirred at 65°C for about 45 minutes. When the reactant became a yellow transparent solution, the Schlank tube was placed in ice water, and the temperature of the reactant was lowered to 0°C. After the temperature of the reactant was lowered, triphenylphosphine (Triphenylphosphine, 4 g, 15.28 mmol) was dissolved in 10 ml of tetrahydrofuran (THF), and slowly added dropwise into the Schlenk tube. After complete dropwise addition, the reaction was carried out at 0° C. for 1 hour, and at normal temperature (23° C.) for 1 hour. After the reaction, remove the supernatant, wash the precipitate once with distilled water, wash with ethanol and ether for 3 times and then dry it to obtain the catalyst precursor (Mo(O)(O 2 ) 2 (PPh 3 ) 2 ) (yield: 86%).
[0121] 1 H-NMR (CD 2 Cl ...
Embodiment 2
[0136] Using Mo(O)(O 2 ) 2 (P(OEt) 3 ) 2 Hydrocracking reaction of
[0137] Step 1. In the step 1 of the above-mentioned embodiment 1, as the ligand of the coordination number 1, use triethoxy phosphite (triethylphosphite) instead of triphenylphosphine (triphenylphosphine), in the step of the above-mentioned embodiment 1 1 Under the same reaction conditions, the catalyst precursor (Mo(O)(O 2 ) 2 (P(OEt) 3 ) 2 ).
[0138] 1 H-NMR (DMSO-d 6 ,ppm): 1.23(m,18H),3.98(m,12H)
[0139] 13 C-NMR (DMSO-d 6 ,ppm): 15.90, 15.96, 63.01, 63.07
[0140] FT-IR (cm -1 ): 942,983,911,803
[0141] Step 2. Use the above-mentioned hydrocracking reaction catalyst precursor (Mo(O)(O 2 ) 2 (P(OEt) 3 ) 2 ), carried out the hydrocracking reaction under the same reaction conditions as step 2 of the above-mentioned embodiment 1.
[0142] In addition, each product was analyzed according to the evaluation method of each step performed in Example 1, and the analysis results of the produc...
Embodiment 3
[0144] Using Mo(O)(O 2 ) 2 (PCy 3 ) 2 Hydrocracking reaction of
[0145] Step 1. In step 1 of the above-mentioned embodiment 1, as a ligand with a coordination number of 1, tricyclohexanephosphine (tricyclohexanephosphine) is used instead of triphenylphosphine (triphenylphosphine), which is the same as step 1 of the above-mentioned embodiment 1 The catalyst precursor (Mo(O)(O 2 ) 2 (PCy 3 ) 2 ).
[0146] 1 H-NMR (CD 2 Cl 2,ppm): 1.99(m,9H), 1.81(d,6H), 1.68(s,3H), 1.51(t,6H), 1.26(s,9H)
[0147] 13 C-NMR (CD 2 Cl 2 , ppm): 217.19 (Mo-CO), 37.72 (P-C-), 30.43 (-CH 2 -)
[0148] Step 2. Use the above-mentioned hydrocracking reaction catalyst precursor (Mo(O)(O 2 ) 2 (PCy 3 ) 2 ), under the same reaction conditions as step 2 of the above-mentioned embodiment 1, carry out the hydrocracking reaction.
[0149] In addition, each product was analyzed according to the evaluation method of each step performed in Example 1, and the analysis results of the product pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com