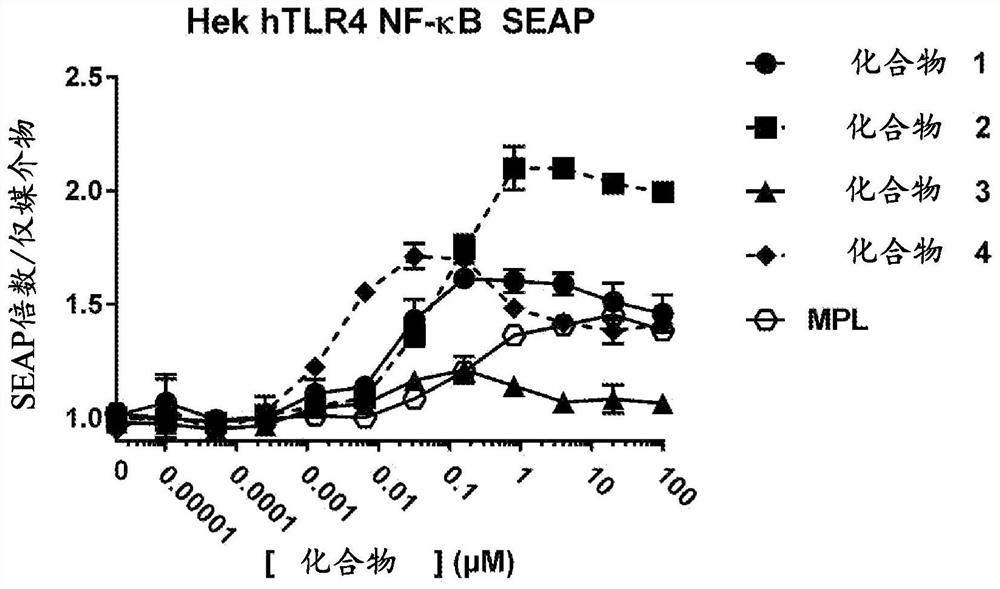
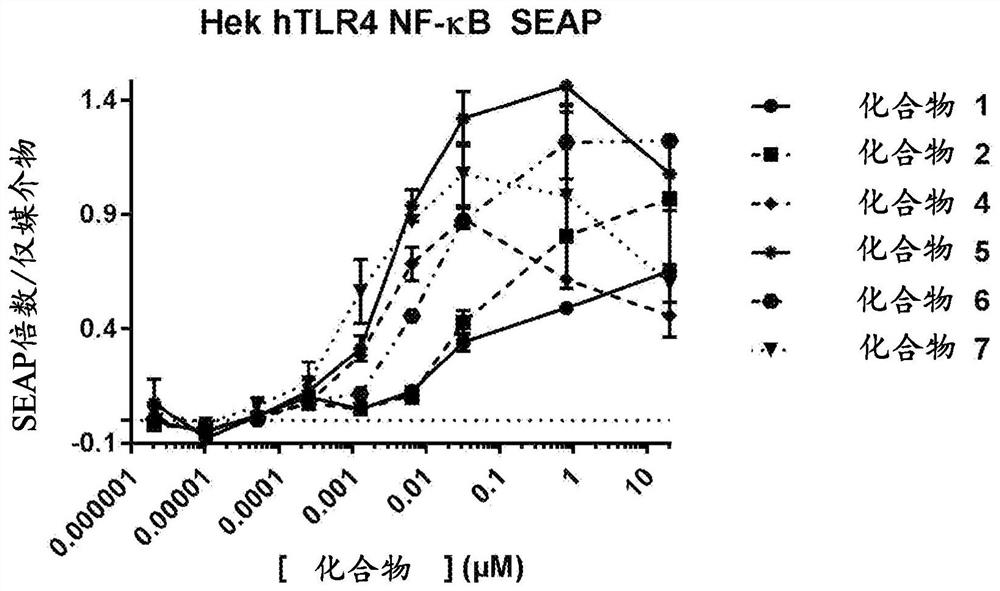
Toll-like receptor ligands
A technology of -O-C, -OSO3H, applied in pharmaceutical formulations, antibody medical components, viruses/bacteriophages, etc., can solve problems such as low efficacy, hindered use, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0325] 2-[(R)-3-Decanoyloxytetradecanoylamino]ethyl 2,3-bis-[(R)-3-Decanoyloxytetradecanoylamino]-2,3-di Preparation of deoxy-4-O-phosphono-β-D-allopyranoside (allopyranoside) (compound 1)
[0326]
example 1
[0329] 1,3,4,6-Tetra-O-acetyl-2-amino-2-deoxy-β-D-glucopyranose hydrochloride (76.47 g, 0.23 mol) was dissolved in dichloromethane (350 mL) and H 2 The solution in O (350 mL) was treated with sodium bicarbonate (149.94 g, 1.79 mol) added slowly in portions. Benzyl chloroformate (79.17 g, 0.46 mol) was added portionwise to control gas evolution and the reaction was stirred vigorously for 2.5 hours. The layers were separated, and the aqueous layer was extracted with dichloromethane (100 mL). The combined organic layers were washed with saturated aqueous sodium chloride, dried over anhydrous sodium sulfate, filtered and concentrated to about 100 mL. Methyl tert-butyl ether (200 mL) was added and the resulting mixture was stirred and cooled to 0 °C and the precipitate was collected by filtration, washed with cold methyl tert-butyl ether and dried in a vacuum oven to give 88.89 g (81%) of 1,3,4,6-tetra-O-acetyl-2-(benzyloxycarbonylamino)2-deoxy-β-D-glucopyranoside.
example 1
[0331] The compound prepared in Example 1A above (10 g, 20.8 mmol) and benzyl N-(2-hydroxyethyl)carbamate (4.48 g, 22.9 mmol) cooled to -15 °C were dissolved in anhydrous dichloromethane (80 mL) The solution in , was treated dropwise with trimethylsilyl triflate (0.37 mL, 2.08 mmol). The reaction mixture was allowed to warm to room temperature over 5.5 hours. The reaction was quenched with saturated aqueous sodium bicarbonate (40 mL), and the layers were separated. The aqueous layer was extracted with dichloromethane (2 x 20 mL), and the combined organic layers were dried over anhydrous sodium sulfate, filtered and concentrated in vacuo. The crude product obtained was crystallized from dichloromethane / heptane to give 10.4 g (81%) of 2-(benzyloxycarbonylamino)ethyl 3,4,6-tri-O-acetyl-2- Benzyloxycarbonylamino-2-deoxy-β-D-glucopyranoside as a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com