Anti-cancer tetravalent platinum complex capable of inhibiting inflammation and immune escape and preparation method thereof
A technology for immune escape and anti-inflammation, applied in the field of biomedicine, which can solve the problems of low selectivity and high systemic toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
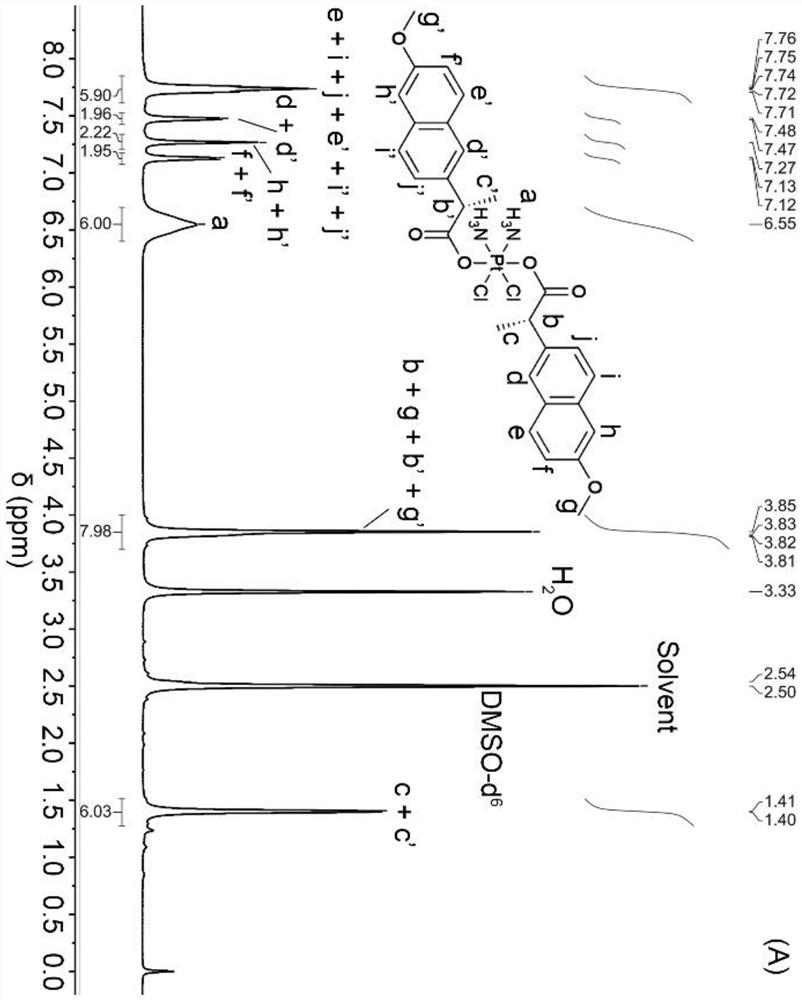
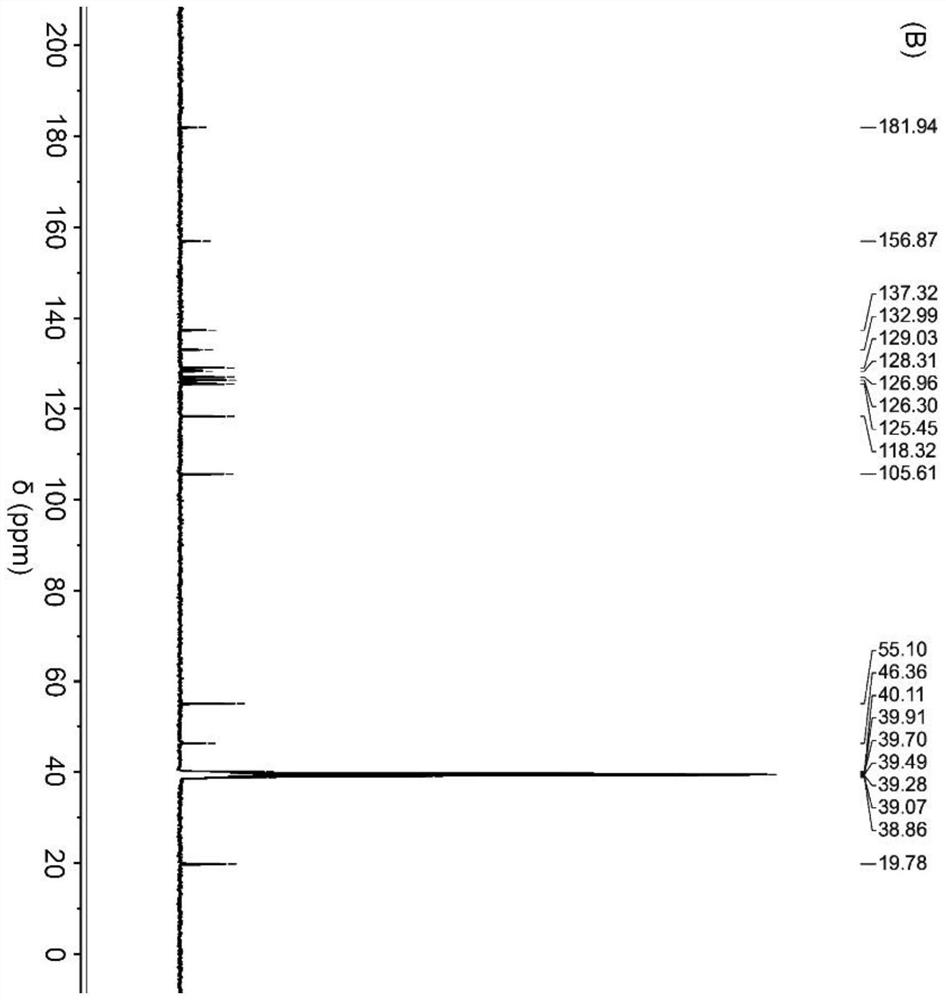
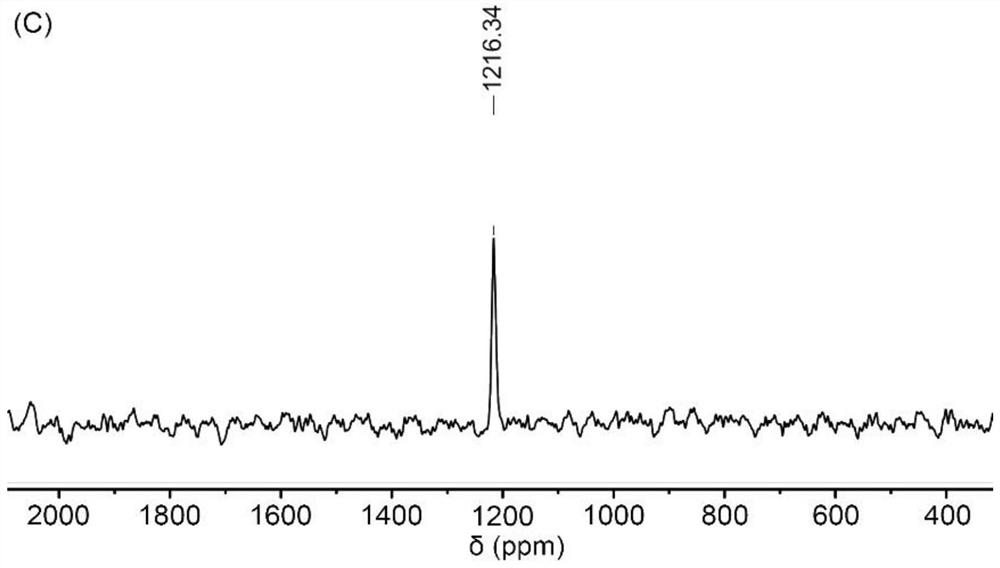
[0032] Synthesis of Tetravalent Platinum Complexes
[0033]
[0034] S1. Add hydrogen peroxide (30wt%, 20mL) dropwise into an aqueous solution (12mL) of cisplatin (CDDP, 400mg, 1.33mmol), and stir under reflux at 60°C for 4h. Cool overnight to obtain pale yellow crystals, filter and wash with ice water, and dry in vacuo to obtain tetravalent Oxoplatin yellow powder product;
[0035] S2, combine oxoplatin (100mg, 0.30mmol) with (NPX, 207.23 mg, 0.90 mmol), TEA (125 μL, 0.90 mmol) and TBTU (288.99 mg, 0.90 mmol) were mixed in DMF (15 mL), and stirred for 48 h in the dark.
[0036] S3. After the reaction is completed, a mixture of ethanol and water is added to the mixture, and a yellow crude product is precipitated. The precipitate was dissolved in methanol, washed several times with ether, and dried in vacuum to obtain a light yellow powder with a yield of about 65%.
Embodiment 2
[0037] The cytotoxic activity test of embodiment 2 compound
[0038] The compounds prepared in Example 1 of the present invention were tested for their cytotoxic activity, using breast cancer cells (MFC-7, MDA-MB-231, MDA-MB-435), ovarian cancer cells (Caov-3) and cervical cancer cells ( HeLa) as a model, with the compound synthesized in Example 1 and cisplatin as the test substance, after the test substance acts on the cells, observe the survival situation of the cells, test the cell survival rate with the thiazolyl blue (MTT) method, The specific operation steps are as follows:
[0039] S1. Collect the above-mentioned logarithmic phase cells, adjust the concentration of the cell suspension, add them to a 96-well plate, and the number of cells per well is about 5000;
[0040] S2. Place the above experimental cells at 37°C, 5% CO 2 Cultivated in a cell culture incubator for 18h;
[0041] S3. Compounds or cisplatin solutions with different concentration gradients were prepar...
Embodiment 3
[0050] Anti-inflammatory ability detection of the compound of embodiment 3
[0051] The anti-inflammatory ability of the compound prepared in Example 1 of the present invention was tested, mouse macrophage RAW264.7 and human breast cancer cell MCF-7 were selected as models, and the compound synthesized in Example 1 and cisplatin were used as the substances to be detected , after the substances to be detected acted on the cells, the relative content of inflammatory factors IL-6 and IL-1β in the RAW264.7 cell supernatant was detected by ELISA kit, and the COX- 2 The change of protein and the relative content of PGE2 in the cell supernatant, the specific operation steps are as follows:
[0052] S1. Collect the above logarithmic phase cells and adjust the concentration of the cell suspension to 2×10 cells per well. 5 cells at a density of 6 wells;
[0053] S2. Place the above experimental cells at 37°C, 5% CO 2 Cultivated in a cell culture incubator for 18 hours;
[0054] S3. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com