Preparation method of recombinant human glucagon-like peptide-1 analogue fusion protein
A glucagon and fusion protein technology, which is applied in the field of preparation of recombinant human glucagon-like peptide-1 analog fusion protein, can solve the problem of easy degradation of the target protein, low recovery rate of effective activity, and instability of fusion protein products And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Construction of Engineering Bacteria Expressing Recombinant Human Glucagon-Like Peptide-1 Analogue Fusion Protein
[0055] According to the method of CN201310331182.6 invention patent application, the GLP-1 analog fusion protein expression engineering bacteria were prepared. In short, the N-terminal part of the GLP-1 analog-E3-HSA gene fragment was artificially synthesized, the HSA coding gene sequence was obtained by PCR amplification, the GLP-1 analog-E3-HSA fusion gene was obtained by overlapping PCR, and its sequence was identified by sequencing As shown in SEQ ID NO:5. The GLP-1 analogue-E3-HSA / pPIC9 recombinant plasmid was obtained by XhoI and NotI double digestion and introduced into the pPIC9 plasmid. The GLP-1 analogue-E3-HSA / pPIC9 recombinant plasmid was linearized with SalI and then electrotransformed into Pichia pastoris GS115 competent cells, screened and identified, and positive transformants were obtained, which were induced and expressed on a...
Embodiment 2
[0056] Example 2: Large-scale fermentation culture and induced expression method of engineering bacteria expressing recombinant human glucagon-like peptide-1 analogue fusion protein
[0057] 1. Preparation of medium
[0058] Weigh 46.5g of calcium sulfate dihydrate, 910.0g of potassium sulfate, 383.0g of magnesium sulfate, 4000.0g of glycerin, measure 1335.0ml of 85% phosphoric acid and add it to a stainless steel bucket, add water to dissolve it, add 207.0g KOH and 20.0ml GPE defoamer to dissolve Afterwards, add purified water to a fixed position in a stainless steel bucket to make a constant volume of 90L.
[0059] 2. Sterilization
[0060] Add the prepared medium into the fermenter with a peristaltic pump, and carry out actual sterilization. The sterilization conditions are 115°C and 30min. After the fermenter is sterilized, the feeding pipeline and transplanting pipeline are sterilized online for 20 minutes with branch steam.
[0061] 3. Preparation before transplantati...
Embodiment 3
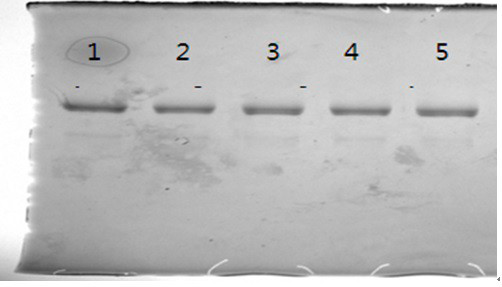
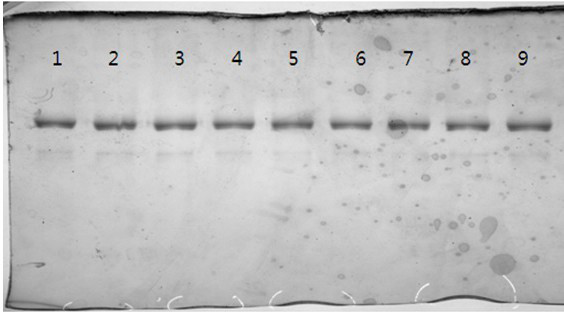
[0074] Example 3: Purification method of recombinant human glucagon-like peptide-1 analogue fusion protein
[0075] 1. Affinity BlUE chromatography
[0076] Packing: packing Blue Sepharose Fast Flow 3.1L, column height 10cm, column BPG 200 / 500.
[0077] Solution: Balance solution BUFFER A (10mmol / L NaAc-HAc pH5.0+5mmol / L EDTA-2Na+0.15mol / L NaCl).
[0078] Washing solution: BUFFER B1 (25% ethylene glycol).
[0079] Eluent: BUFFER B2 (20mmol / L Tris-HCl pH8.0+1mol / L NaCl+25% ethylene glycol+5mmol / L EDTA-2Na).
[0080] First use the balance solution BUFFER A to balance the chromatography column for 2-3CV (column volume), and keep the flow rate at 50-80cm / h throughout the chromatography process. After equilibration, start to load the sample. The load of the sample should be controlled at 6-10mg / ml. After the sample is loaded, first equilibrate the column with 2-2.5CV of BUFFER A, then wash the column with 2-2.5CV of BUFFER B1, and then use 2 ~ 2.5CV of BUFFERB2 elution, when th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com