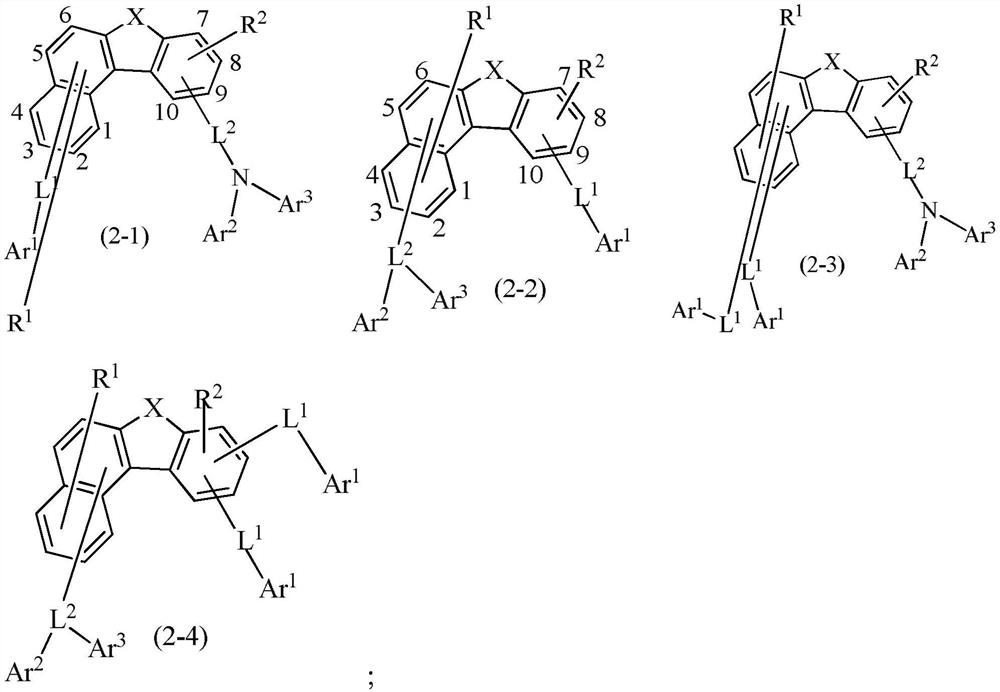
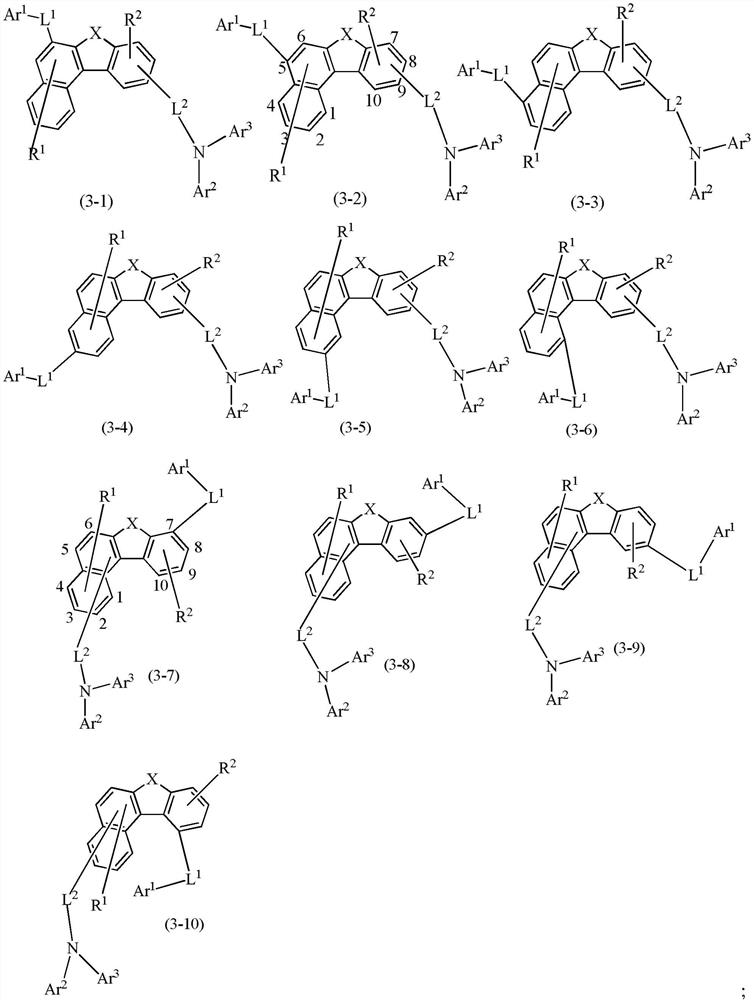
Compounds and application thereof, and organic light-emitting device comprising compound
A technology of compounds and derivatives, applied in the field of organic electroluminescent devices, can solve the problems of stability to be further improved, low driving voltage stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0123]
[0124] (1) Preparation of compound P3-1:
[0125] Add raw materials S1 (31.2g, 100mmol), S2 (31.5g, 100mmol), potassium carbonate (20.7g, 150mmol), tetrakis (triphenylphosphine) palladium 0.5g, add dioxane 300mL, water 50mL, Heated to reflux for 10 h, thin layer chromatography (TLC) detected the completion of the reaction of the raw materials, quenched with water, extracted with dichloromethane, concentrated the organic phase, purified by column chromatography to obtain the product, and obtained the target compound P3-1.
[0126] (2) Preparation of Compound P3
[0127] Compound P3-1 (27.3g, 50mmol), diphenylamine (10.1g, 60mmol), sodium tert-butoxide (9.6g, 100mmol), tris(dibenzylideneacetone) dipalladium 0.5g and 2-dicyclohexyl Add 0.5 g of phosphine-2′,6′-dimethoxy-biphenyl into a flask containing toluene (300 mL), heat to reflux for 5 hours, and monitor the completion of the reaction by thin-layer chromatography (TLC). After cooling down, the reaction solution ...
preparation example 2
[0131]
[0132] (1) Preparation of compound P9-1:
[0133] Add raw materials S1 (100mmol), S3 (100mmol), potassium carbonate (20.7g, 150mmol), tetrakis(triphenylphosphine)palladium 0.5g into the one-mouth bottle, add dioxane 300mL, water 50mL, heat to reflux for 10h , Thin-layer chromatography (TLC) detected that the reaction of the raw materials was completed, quenched with water, extracted with dichloromethane, concentrated the organic phase and purified by column chromatography to obtain the product, and the target compound P9-1 was obtained.
[0134] (2) Preparation of Compound P9
[0135]Compound P9-1 (60mmol), triphenylamine 3-boronate (60mmol), potassium carbonate (100mmol), tris(dibenzylideneacetone) dipalladium 0.5g and 2-dicyclohexylphosphine-2',6'- Add 0.5 g of dimethoxy-biphenyl into a flask containing 300 mL of dioxane and 50 mL of water, heat to reflux for 10 h, monitor the completion of the reaction by thin-layer chromatography (TLC), extract with dichlorome...
preparation example 3
[0139]
[0140] (1) Preparation of compound P34-1:
[0141] Add raw materials S4 (100mmol), S5 (110mmol), sodium tert-butoxide (150mmol), 0.5g of tris(dibenzylideneacetone)dipalladium and 2-dicyclohexylphosphine-2',6'-di Add 0.5 g of methoxy-biphenyl into a flask containing toluene (300 mL), heat to reflux for 5 h, and thin-layer chromatography (TLC) detects that the raw materials have reacted. The purified pale yellow solid P34-1 was analyzed.
[0142] (2) Preparation of compound P34-2
[0143] Compound P34-1 (60mmol), potassium acetate 100mmol, bipinacol borate 120mmol, tris(dibenzylideneacetone) dipalladium 0.5g and 2-dicyclohexylphosphine-2',6'-dimethyl Add 0.5 g of oxy-biphenyl to 300 ml of dioxane, reflux for 6 hours, thin-layer chromatography (TLC) detects that the raw materials have reacted, post-treatment, add water and ethyl acetate to extract, separate, and concentrate the organic phase to obtain a white solid P34-2.
[0144] (3) Preparation of Compound P34 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com