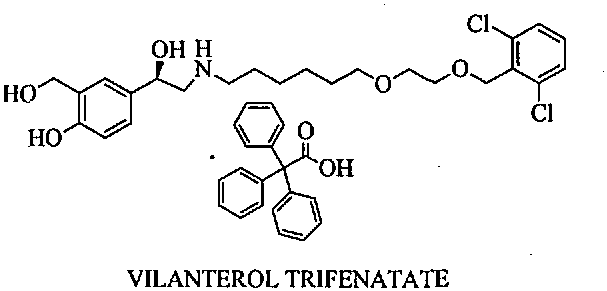
Preparation method of vilanterol and salt thereof
A compound and mixture technology, applied in the field of preparation of vilanterol and its salts, can solve the problems of high cost, low yield, low purity, etc., and achieve the effect of low cost, high yield and high quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
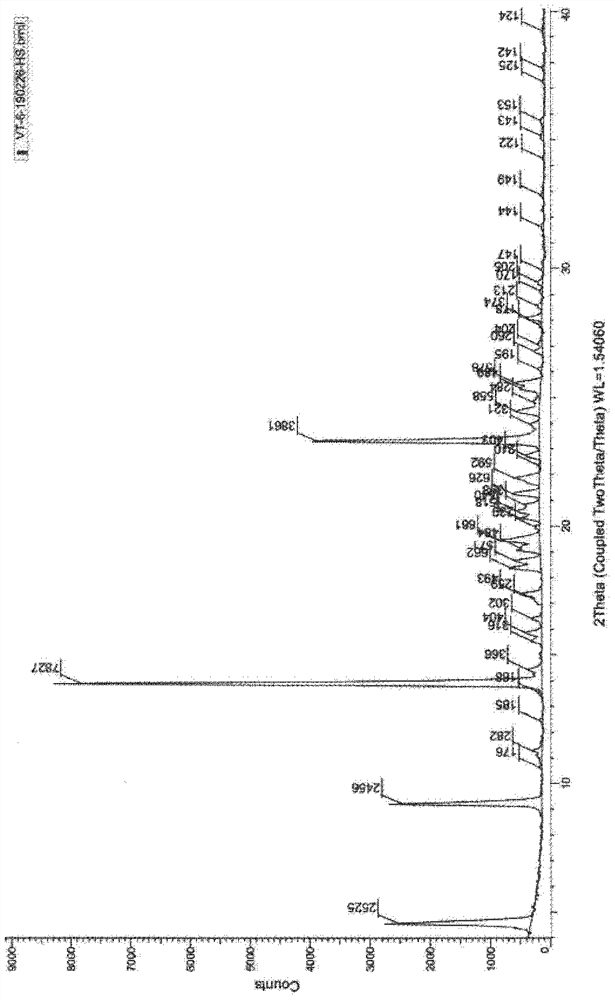
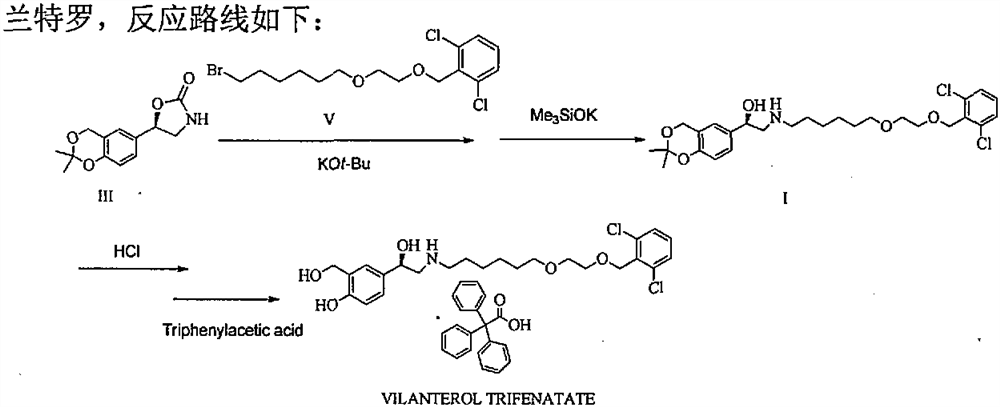
[0050] Add (5R)-5-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)-1,3-oxazolidine-2 to a 1L reaction flask - Ketone (15.0 g, S isomer content 0.5%) and N, N- dimethylformamide 150 ml, stirred and dissolved, cooled to 5 ° C in an ice bath, added potassium tert-butoxide (10.1 g), stirred at room temperature for 1 hour, then added 2-[2-(6-bromo-hexyloxy)-ethoxymethyl]-1,3-dichloro-benzene (27.6g) to the solution, stirred at room temperature for 24 hours, TLC The detection response is complete. Join H 3 PO 4 / KH 2 PO 4 Buffer solution, adjust the pH to about 7, stir for 10 minutes, extract 3 times with ethyl acetate, combine the organic phases, wash 2 times with water, and concentrate the organic phases under reduced pressure to obtain a crude product of pale yellow oil.
[0051] At room temperature, tetrahydrofuran (300ml) was added to the crude oil, followed by potassium trimethylsiliconate (23.1g), heated to reflux, stirred for 3h, and the reaction was complete by TLC. The reactio...
Embodiment 2
[0057] Add (5R)-5-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)-1,3-oxazolidine-2 to a 1L reaction flask - Ketone (15.0 g, S isomer content 0.5%) and N, N- dimethylformamide 150 ml, stirred and dissolved, cooled to 5 ° C in an ice bath, added potassium tert-butoxide (10.1 g), stirred at room temperature for 1 hour, then added 2-[2-(6-bromo-hexyloxy)-ethoxymethyl]-1,3-dichloro-benzene (34.5g) to the solution, stirred at room temperature for 24 hours, TLC The detection response is complete. Join H 3 PO 4 / KH 2 PO 4 Buffer solution, adjust the pH to about 7, stir for 10 minutes, extract 3 times with ethyl acetate, combine the organic phases, wash 2 times with water, and concentrate the organic phases under reduced pressure to obtain a crude product of pale yellow oil.
[0058] At room temperature, tetrahydrofuran (300ml) was added to the crude oily product, followed by potassium trimethylsiliconate (38.5), heated to reflux, stirred and reacted for 3h, and the reaction was complete ...
Embodiment 3
[0060] Add (5R)-5-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)-1,3-oxazolidine-2 to a 1L reaction flask - Ketone (15.0 g, S isomer content 0.5%) and N, N- dimethylformamide 150 ml, stirred and dissolved, cooled to 5 ° C in an ice bath, added potassium tert-butoxide (10.1 g), stirred at room temperature for 1 hour, then added 2-[2-(6-bromo-hexyloxy)-ethoxymethyl]-1,3-dichloro-benzene (25.3g) to the solution, stirred at room temperature for 24 hours, TLC The detection response is complete. Join H 3 PO 4 / KH 2 PO 4 Buffer solution, adjust the pH to about 7, stir for 10 minutes, extract 3 times with ethyl acetate, combine the organic phases, wash 2 times with water, and concentrate the organic phases under reduced pressure to obtain a crude product of pale yellow oil.
[0061] At room temperature, tetrahydrofuran (300ml) was added to the crude oily product, followed by potassium trimethylsiliconate (7.7g), heated to reflux, stirred and reacted for 6h, and the reaction was complete ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com