Composition for degrading opioid peptide
A technology of opioid peptides and compositions, applied in the field of opioid peptide decomposing agents or opioid peptide decomposing compositions, to achieve the effect of less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
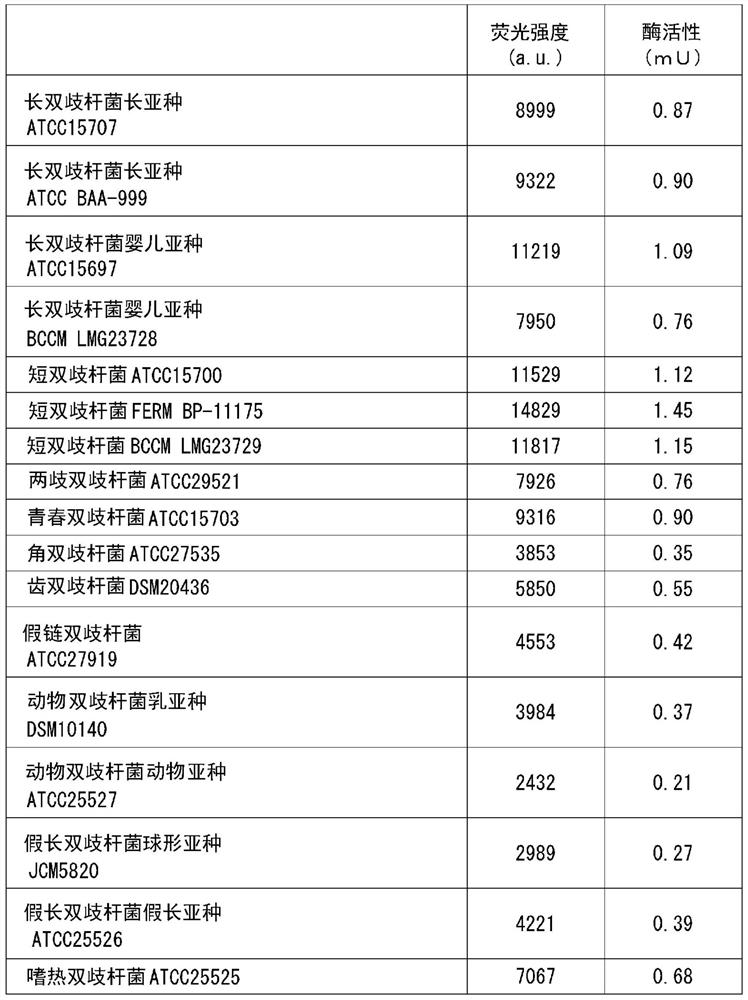
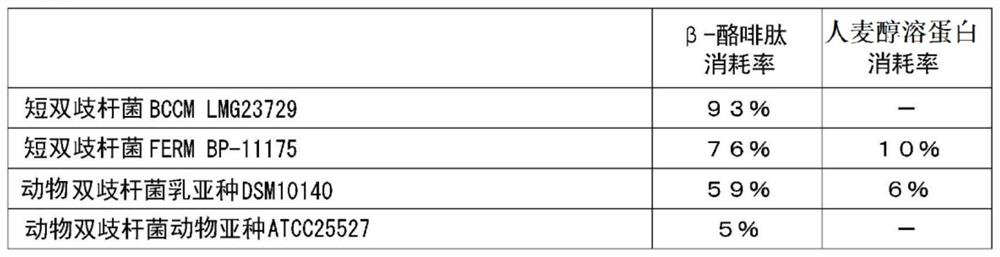
[0206] One or more of the 17 kinds of Bifidobacteria bacteria used in Test Example 1 were added or added to 3 mL of the same MRS liquid medium, and anaerobic culture was carried out at 37 ° C for 16 hours, and the culture solution Concentrate and freeze-dry to obtain the end of one or more bacteria. The powder of one or more fungi is mixed with excipients and the like to form a tablet. The intake of bacteria in total is 1×10 6 ~1×10 12 The tablet was ingested daily for 3 months in the form of cfu / kg body weight / day.
[0207] The opioid peptide decomposition effect can be expected by ingestion of this tablet.
manufacture example 2
[0209] One or more of the 17 kinds of Bifidobacterium bacteria used in Test Example 1 were added or added to 3 mL of the same MRS liquid medium, and anaerobic culture was carried out at 37 ° C for 16 hours, and the culture solution was concentrated. Freeze-drying is carried out to obtain the end of one or more bacteria. Fermented milk was obtained by adding the one or more types of powdered bacteria to fermented milk raw materials. The intake of bacteria in total is 1×10 6 ~1×10 12 The fermented milk was ingested every day for at least 3 months in the form of cfu / kg body weight / day.
[0210] The opioid peptide decomposition effect can be expected by ingestion of this fermented milk.
manufacture example 3
[0212] The manufacturing method of the formula milk powder which added 1 or more types of bacteria selected from the 17 types of Bifidobacterium genus bacteria used in Test Example 1 is shown below.
[0213] Desalted milk whey protein powder (manufactured by Mirai Co., Ltd.) 10 kg, milk casein powder (manufactured by Fonterra Co., Ltd.) 6 kg, lactose (manufactured by Mirai Co., Ltd.) 48 kg, mineral mixture (manufactured by Tomita Pharmaceutical Co., Ltd.) 920 g, vitamin mixture (manufactured by Tanabe Pharmaceutical Co., Ltd.) Dissolve 32 g of lactulose (manufactured by Morinaga Dairy Co., Ltd.), 500 g of raffinose (manufactured by Nippon Beet Sugar Manufacturing Co., Ltd.), and 900 g of galactooligosaccharide liquid sugar (manufactured by Yakult Pharmaceutical Industry Co., Ltd.) In 300 kg of warm water, it was heated and dissolved at 90° C. for 10 minutes, and 28 kg of prepared fat (manufactured by Sun Oil Co., Ltd.) was added and homogenized. Then, sterilizing, concentratin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com