Preparation method of prostaglandin analogue
A prostaglandin and analogue technology, which is applied in the field of preparation of prostaglandin analogues, can solve the problems of low yield, fewer prostaglandin synthesis methods, and harsh conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
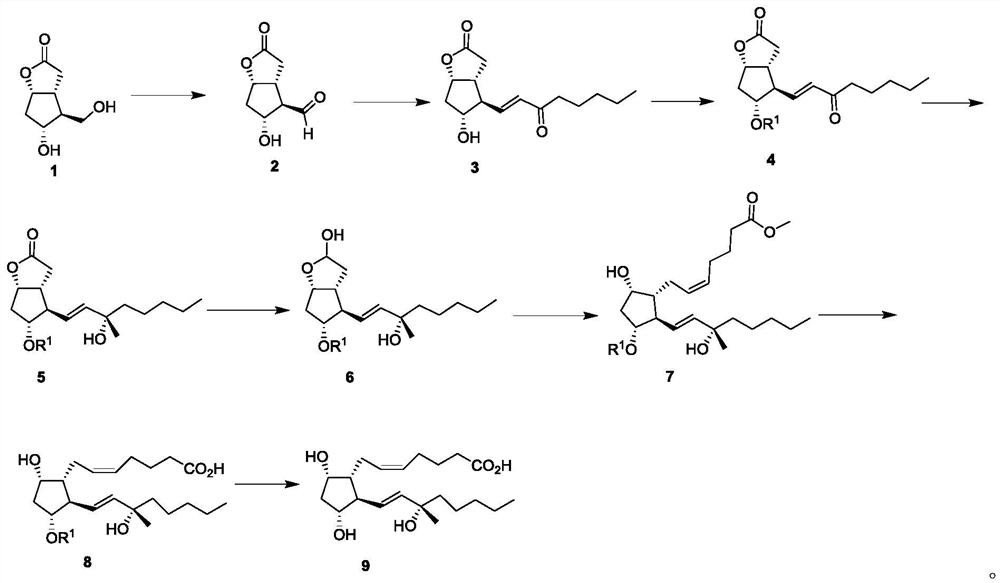
[0091] (1) Synthesis of compound 2
[0092]
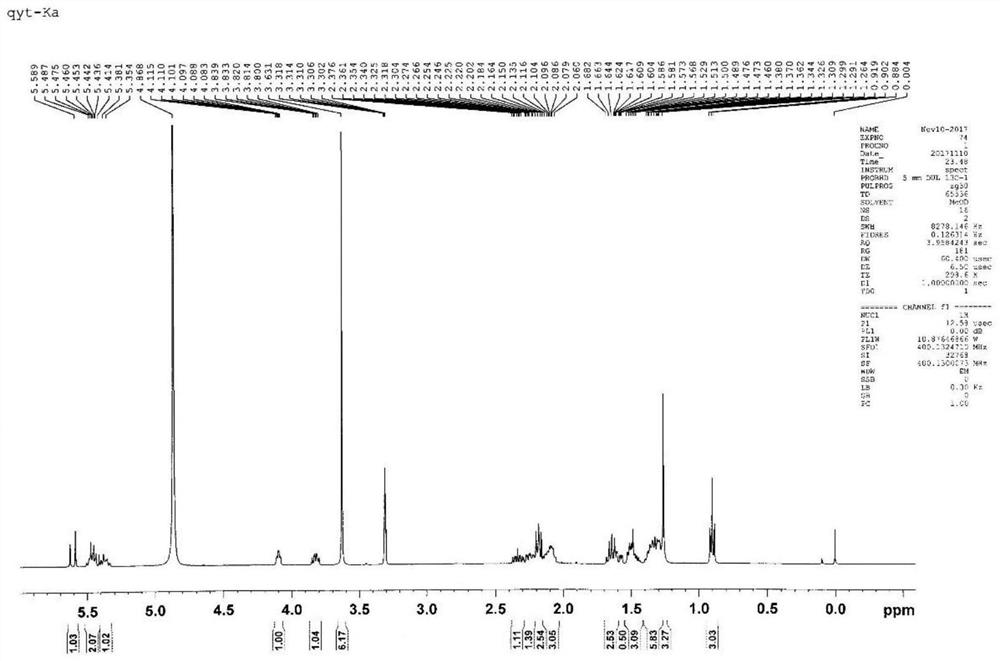
[0093] Compound 1 (5.7g, 30mmol) and TEMPO (470mg, 3mmol) were dissolved in 500mL DMA / DCM (v / v=1:300), at room temperature, PhI(OAc) 2 (10.6g, 33mmol) was slowly added to the above solution. The reaction solution was stirred at room temperature for 6 h. The solvent was distilled off under reduced pressure to obtain a black oily crude product without any residual acetic acid, which was directly put into the next reaction without purification. 1 H NMR (400MHz, Chloroform-d) δ9.44 (d, J = 6.2Hz, 1H), 4.82 (dt, J = 5.1, 4.2Hz, 1H), 3.94 (qd, J = 5.6, 4.6Hz, 1H) ,3.61(d,J=5.3Hz,1H),2.67(q,J=5.9Hz,1H),2.65–2.50(m,3H),2.16(dt,J=14.5,5.4Hz,1H),1.83– 1.75(m,1H).
[0094] (2) Synthesis of compound 3
[0095]
[0096] 0°C, under the protection of nitrogen, the solution of β-dimethoxyheptyl carbonyl phosphate (10g, 45mmol) in THF (50mL) was slowly added dropwise to the suspension of NaH (1.8g, 45mmol) in anhydrous THF (100mL) middl...
Embodiment 2
[0119] (1) Synthesis of compound 2
[0120]
[0121] A solution of DMSO (3ml, 42.2mmol) in DCM (20mL) was added dropwise to a solution of oxalyl chloride (1.5ml, 17.7mmol) in DCM (20mL) at -78°C. After stirring for 30 min, a solution of compound 1 (1.80 g, 10.5 mmol) in DCM (15 mL) was added dropwise to the above solution, and stirred for 2 min. Stirring was maintained at -78°C for 1 h, then TEA (12 mL, 86.0 mmol) was added dropwise to the above reaction solution. After the addition of TEA is complete, add saturated NH 4 Cl solution (10 mL) quenched the reaction. Then, the temperature of the reaction solution was raised to room temperature, washed with saturated brine (3×20mL), and the organic phase was anhydrous Na 2 SO 4 It was dried, filtered, concentrated, and purified by column chromatography to obtain compound 2 (1.7 g, 95%). 1 H NMR (400MHz, Chloroform-d) δ9.44 (d, J = 6.2Hz, 1H), 4.82 (dt, J = 5.1, 4.2Hz, 1H), 3.94 (qd, J = 5.6, 4.6Hz, 1H) ,3.61(d,J=5.3Hz,1H),...
Embodiment 3
[0147] (1) Synthesis of Compound 2
[0148]
[0149] Dess-Martin oxidant (19 g, 45 mmol) was added portionwise to a solution of compound 1 (5.7 g, 30 mmol) in DCM (200 mL) at 0°C. Then, the reaction was transferred to room temperature, and the reaction was stirred for 2h. After the reaction is complete, place it at 0°C, add saturated sodium thiosulfate solution to quench the reaction, filter with suction, and then add saturated NaHCO to the filtrate 3 solution (50mL) and ethyl acetate (100mL), the layers were separated after standing, the aqueous phase was extracted with ethyl acetate (100mL×2), the organic phases were combined, washed with saturated brine (100mL), and the organic phase was anhydrous Na 2 SO 4 It was dried, filtered, concentrated, and purified by column chromatography to obtain compound 2 (3.8 g, 75%). 1 H NMR (400MHz, Chloroform-d) δ9.44 (d, J = 6.2Hz, 1H), 4.82 (dt, J = 5.1, 4.2Hz, 1H), 3.94 (qd, J = 5.6, 4.6Hz, 1H) ,3.61(d,J=5.3Hz,1H),2.67(q,J=5.9Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com