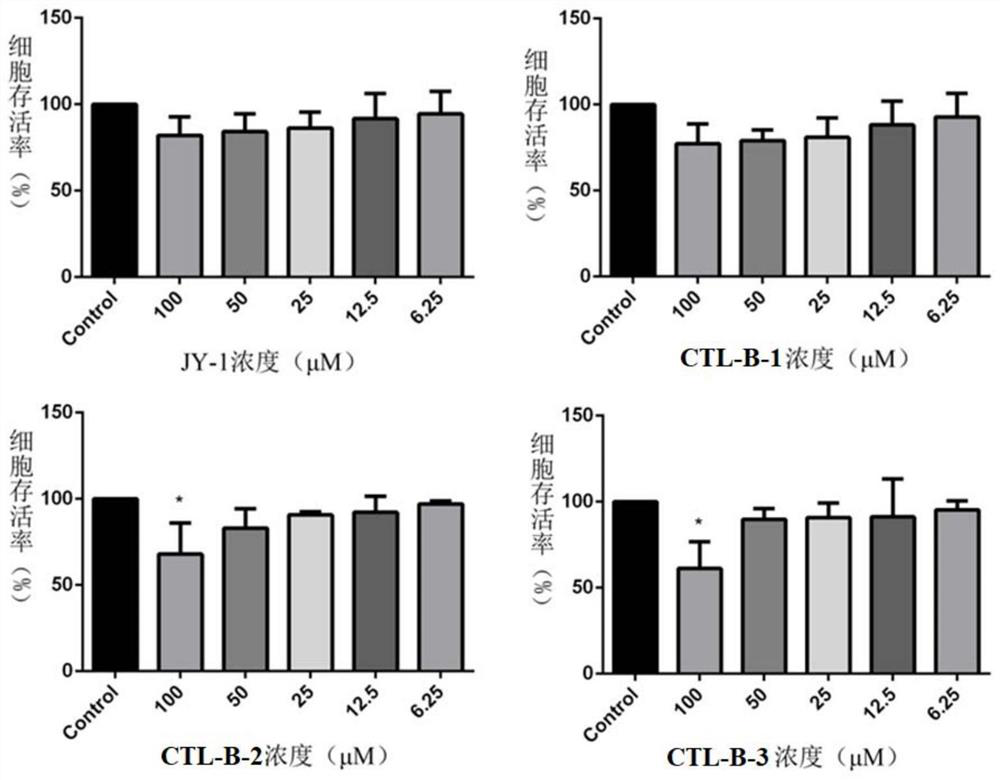
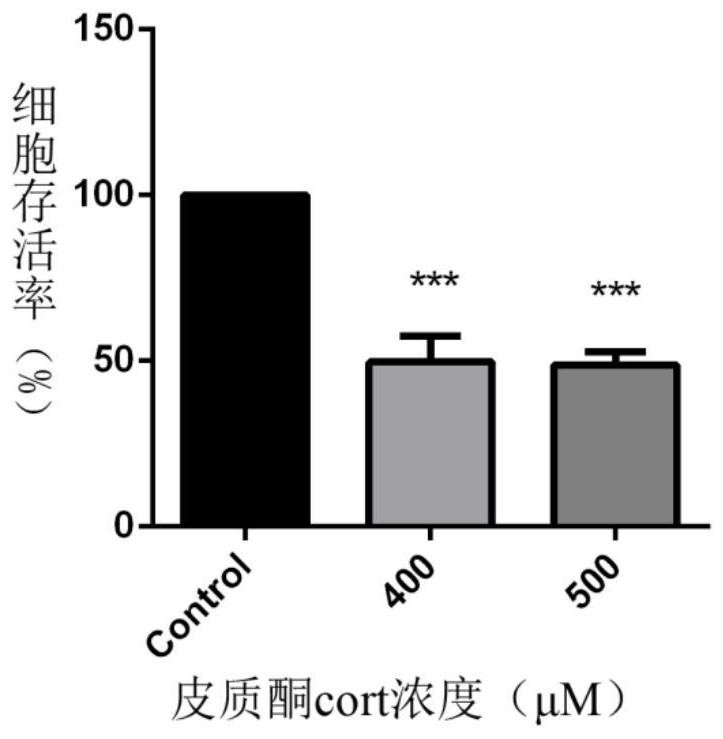
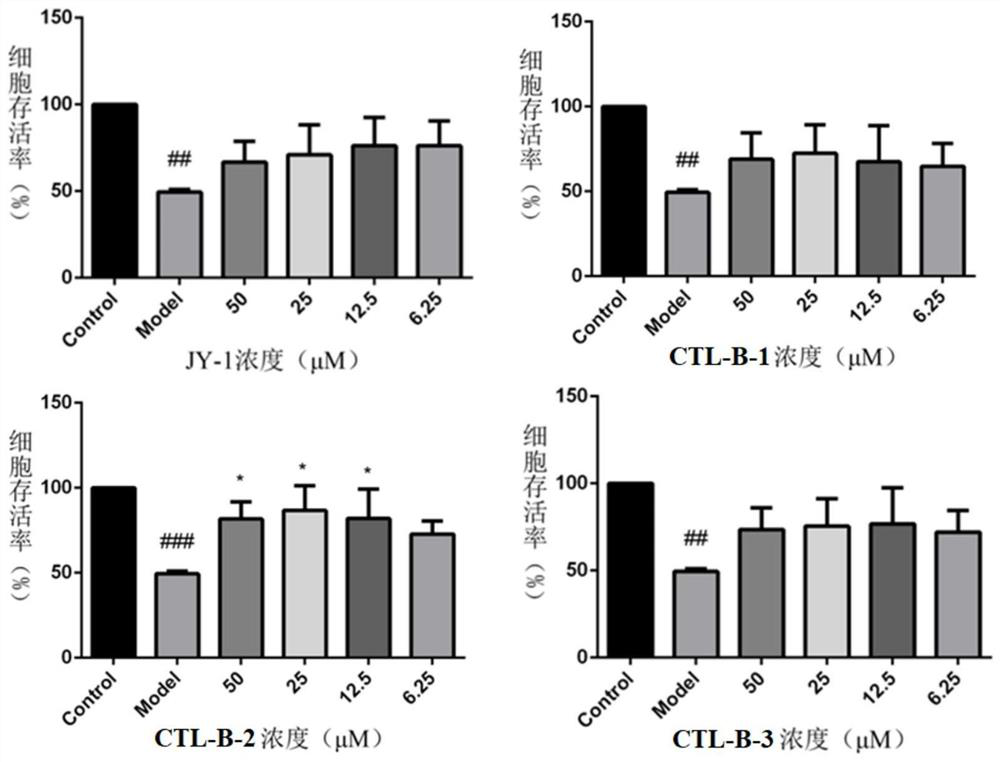
Dihydroartemisinin/neurotransmitter conjugate as well as synthesis method and application thereof
A technology of dihydroartemisinin and neurotransmitters, which is applied in nervous system diseases, organic chemical methods, medical preparations containing active ingredients, etc., to achieve the effect of easy industrialization and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The synthesis of 12-β-O-(N-(β-aminopropionyl))aminoethyldihydroartemisinin (CTL-B-1) comprises the following five steps:
[0053] (1) Preparation of 12-β-O-(2-bromoethyl) dihydroartemisinin: Dissolve (310mg, 1.1mmol) dihydroartemisinin in 20mL dry DCM, add a small amount of activated Molecular sieves, after precooling in ice bath for 5min, add phosphotungstic acid (160mg, 0.05equiv), after activation for 10min, add 2-bromoethanol (206.2mg, 1.65mmol), react in ice bath for 0.5h, room temperature ( 15°C) for 26 hours, followed by TLC. After the reaction, water was added, extracted with DCM, dried, and separated by column chromatography [V (petroleum ether): V (ethyl acetate) = 40:1] to obtain 274.6 mg of a white solid, Yield 63.8%.
[0054] The structural data of the product are as follows: 1 H NMR (CDCl 3 ,300MHz)δ:0.87(d,J=7.5Hz,3H), 0.92(d,J=6.3Hz,3H),1.17-1.26(m,2H),1.40(s,3H),1.57-1.63(m ,1H), 1.66-1.75(m,2H),1.79-1.90(m,1H),1.95-2.02(m,1H),2.02-2.11(m,2H),2.33(...
Embodiment 2
[0064] The synthesis of 12-β-O-(N-(γ-aminobutyryl))aminoethyldihydroartemisinin (CTL-B-2) comprises the following five steps:
[0065] (1) Preparation of 12-β-O-(2-bromoethyl)dihydroartemisinin: the steps are the same as in Example 1 step (1);
[0066] (2) Preparation of 12-β-O-(2-azidoethyl)dihydroartemisinin: the steps are the same as step (2) in Example 1;
[0067] (3) Preparation of 12-β-O-(2-aminoethyl)dihydroartemisinin: the steps are the same as step (3) in Example 1;
[0068] (4) Preparation of 12-β-O-(N-(N-Fmoc-γ-aminobutyryl)aminoethyl)dihydroartemisinin: Weigh Fmoc-γ-butyric acid (325.4mg, 1.0mmol ), implemented according to Example 1 step (4), to obtain 483.7 mg of light yellow oil, yield 76.2%.
[0069] The structural data of the product are as follows: 1 H NMR (300HMz, CDCl 3 )δ:7.76-7.73(m,2H,H-29,H-29a),7.59-7.57(m,2H,H-26,H-26a),7.41-7.36(m,2H,H-28,H -28a),7.31-7.27(m,2H,H-27,H-27a),5.37(d,2H,H-12,H-5),4.77-4.36(m,4H,H-23,H- 24),4.19 (m,1H,H-24),3.79-3.6...
Embodiment 3
[0074] The synthesis of 12-beta-O-(N-(4-(1H-imidazolyl)) aminoethyl dihydroartemisinin (CTL-B-3) comprises the following two steps:
[0075] (1) Preparation of 12-β-O-(2-bromoethyl)dihydroartemisinin: the steps are the same as in Example 1 step (1);
[0076] (2) Preparation of 12-β-O-(N-(4-(1H-imidazolethyl))aminoethyldihydroartemisinin: take 12-β-(O-2-bromoethyl)dihydro Artemisinin (391.3mg, 1.0mmol) was dissolved in 30ml of DMF, histamine (221.4mg, 1.5mmol) and potassium carbonate (414.6mg, 3.0mmol) were added, stirred at 78°C for 3 hours, TLC (DCM: Methanol=10:1, V:V, R f =0.42) monitoring, the reaction was completed, moved to room temperature, extracted with DCM (3×50 ml), dried over anhydrous sodium sulfate, filtered, and concentrated by distillation under reduced pressure to obtain a mixture. Column chromatography separation (DCM:methanol=50:1, V:V,R f =0.15), to obtain 279.1 mg of a yellow oily substance, with a yield of 66.2%.
[0077] The structural data of the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com