Method for preparing Setmelanotide
A technology of resin and amino resin, which is applied in the field of preparation of polypeptide drugs, can solve the problems of complex and cumbersome methods, inconvenient large-scale production, etc., and achieve the effect of simple process operation, high product yield, and simple preparation process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis of embodiment 1 Setmelanotide peptide resin
[0029] Setmelanotide peptide resins are:
[0030] Ac-Arg(Pbf)-Cys(Trt)-D-Ala-His(Trt)-D-Phe-Arg(Pbf)-Trp(Boc)-Cys(Trt)-aminoresin
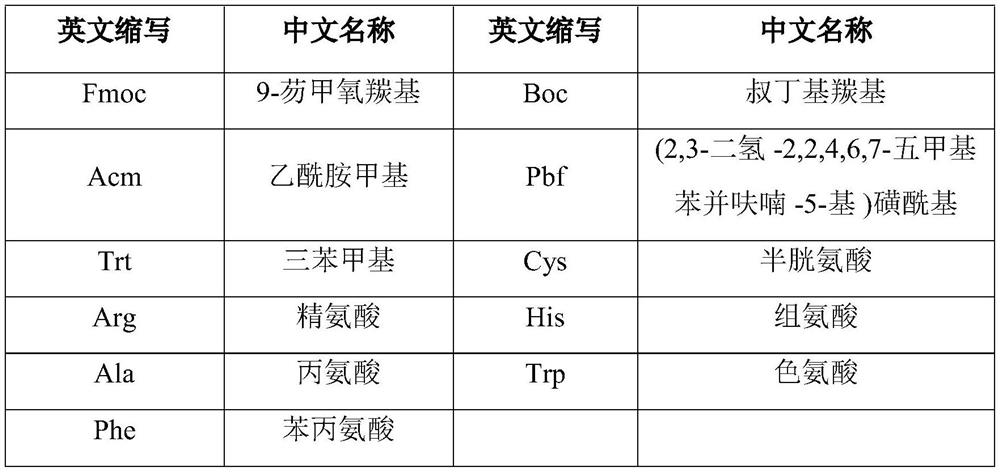
[0031] Using Rink Amide-MBHA resin as the starting resin, the Setmelanotide peptide resin was prepared by de-Fmoc protection and coupling reaction, followed by coupling with the protected amino acids shown in the table below.
[0032] The protected amino acids corresponding to the protected amino acids used in this example are shown in the table below:
[0033] The peptide sequence n= Protected Amino Acids 1 Fmoc-Cys(Trt) 2 Fmoc-Trp(Boc) 3 Fmoc-Arg(Pbf) 4 Fmoc-D-Phe 5 Fmoc-His(Trt) 6 Fmoc-D-Ala 7 Fmoc-Cys(Trt) 8 Fmoc-Arg(Pbf) 9 Ac 2 o
[0034] 1. Insert the first protected amino acid
[0035] Take 0.03 mol of the first protected amino acid and 0.03 mol of HOBt, and dissolve it with an appropriate amount of ...
Embodiment 2
[0040] The synthesis of embodiment 2 Setmelanotide peptide resin
[0041] Setmelanotide peptide resins are:
[0042] Ac-Arg(Pbf)-Cys(Trt)-D-Ala-His(Trt)-D-Phe-Arg(Pbf)-Trp-Cys(Trt)-amino resin
[0043] The preparation method of the peptide resin is the same as in Example 1.
[0044] The protected amino acids corresponding to the protected amino acids used in this example are shown in the table below:
[0045] The peptide sequence n= Protected Amino Acids 1 Fmoc-Cys(Trt) 2 Fmoc-Trp 3 Fmoc-Arg(Pbf) 4 Fmoc-D-Phe 5 Fmoc-His(Trt) 6 Fmoc-D-Ala 7 Fmoc-Cys(Trt) 8 Fmoc-Arg(Pbf) 9 Ac 2 o
Embodiment 3
[0046] The synthesis of embodiment 3 Setmelanotide peptide resin
[0047] Setmelanotide peptide resins are:
[0048] Ac-Arg(Pbf)-Cys(Acm)-D-Ala-His(Trt)-D-Phe-Arg(Pbf)-Trp-Cys(Acm)-amino resin
[0049] The preparation method of the peptide resin is the same as in Example 1.
[0050] The protected amino acids corresponding to the protected amino acids used in this example are shown in the table below:
[0051] The peptide sequence n= Protected Amino Acids 1 Fmoc-Cys(Acm) 2 Fmoc-Trp 3 Fmoc-Arg(Pbf) 4 Fmoc-D-Phe 5 Fmoc-His(Trt) 6 Fmoc-D-Ala 7 Fmoc-Cys(Acm) 8 Fmoc-Arg(Pbf) 9 Ac 2 o
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com