Budesonide-loaded chitosan sponge
A technology of chitosan and sponge, which is applied in the field of chitosan sponge loaded with budesonide, can solve the problems of reducing drug efficacy and dependence on drug efficacy, and achieve the effect of promoting blood coagulation, preventing syneresis, and promoting wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 preparation and performance detection of budesonide-loaded chitosan sponge
[0044] 1. Preparation method
[0045] Dilute 1g of budesonide spray to 20mL with distilled water, add 1g of F127 and stir until dissolved, and configure solution A. Dissolve 10 g of chitosan in 400 mL of distilled water (1% acetic acid) to prepare solution B, then add 5 g of glycerol, 0.5 g of genipin, and solution A, and continue stirring for 60 minutes. The above solution was poured into a silica gel mold, and freeze-dried for storage.
[0046] 2. Scanning electron microscope observation
[0047] S-4800 (Hitachi, Japan) scanning electron microscope was used to observe the microstructure of chitosan hemostatic sponge. All samples were first fully dried at 60°C under vacuum conditions, and sprayed with gold before observation. It can be seen from electron microscope observation that the pore size distribution of the prepared chitosan porous sponge is uniform, about 200 μm. By ...
Embodiment 2
[0048] Embodiment 2 carries the clinical application of budesonide chitosan sponge
[0049] 1 Materials and methods
[0050] 1.1 Clinical data
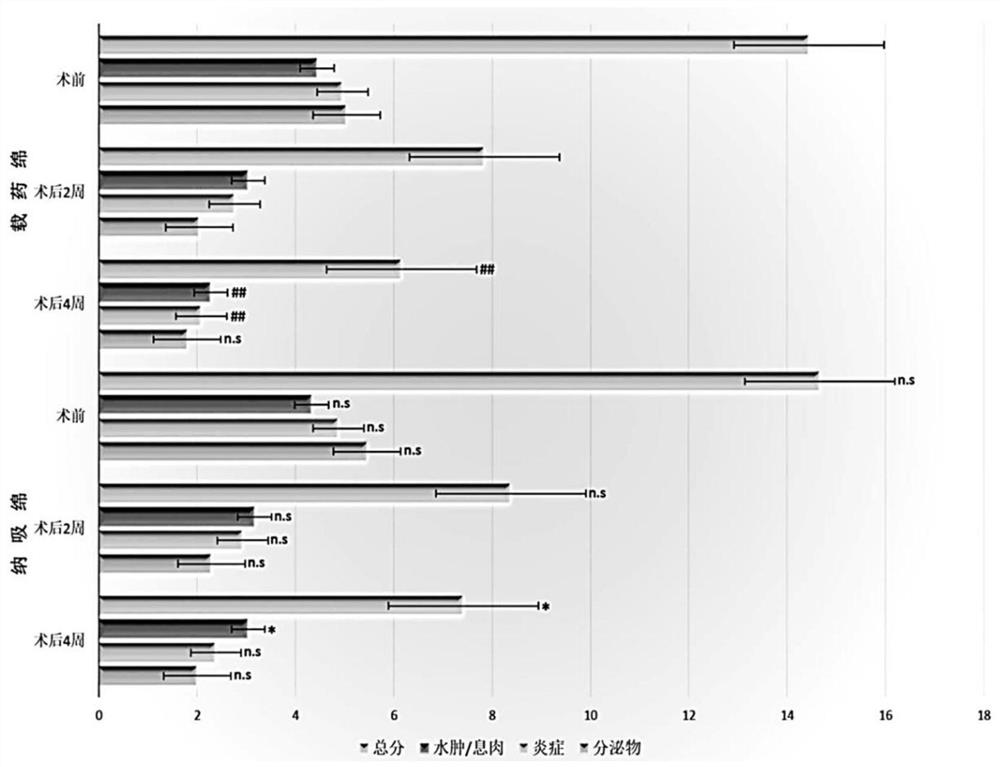
[0051] This study adopts a double-blind, prospective experimental design, and takes CRS patients who received ESS from July 2019 to December 2019 in the Department of Otorhinolaryngology, Affiliated Hospital of Ningbo University School of Medicine as the research object. This research protocol has been approved and filed by the Ethics Committee of Ningbo University School of Medicine. All patients signed informed consent. The diagnostic criteria of CRS refer to the "Guidelines for the Diagnosis and Treatment of Chronic Rhinosinusitis in China (2018)". A total of 49 cases were included in this group of data, including 30 males and 19 females, aged 16-65 years.
[0052] 1.2 Treatment plan
[0053]All patients underwent sinus CT scan and nasal endoscopy before operation. One week before operation, oral antibiotics and budesonide nas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com