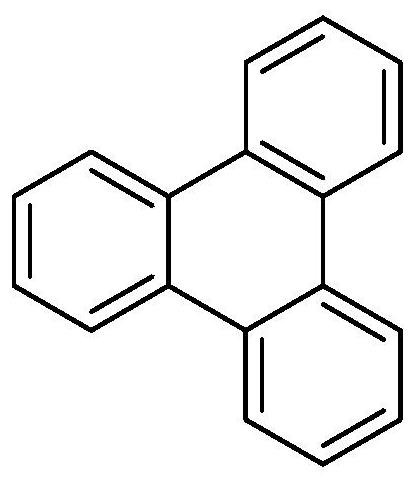
Synthesis method of triphenylene compound
A synthesis method and a triphenylene technology are applied in the field of synthesis of triphenylene compounds, and can solve the problems of harsh reaction conditions, unrecoverable catalysts, etc., and achieve the effects of mild reaction conditions and easy realization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
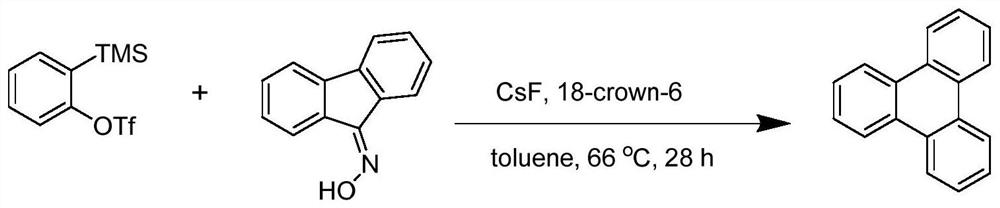
[0027] A kind of synthetic method of triphenylene compound, described synthetic method comprises the following steps:
[0028] Raw material preparation:
[0029] 9-Fluorenone oxime, purity>97%, TCI (Shanghai) Chemical Industry Development Co., Ltd.;
[0030] 2-(Trimethylsilyl)phenyltrifluoromethanesulfonate, purity>95%, TCI (Shanghai) Chemical Industry Development Co., Ltd.
[0031] Specific synthesis process:
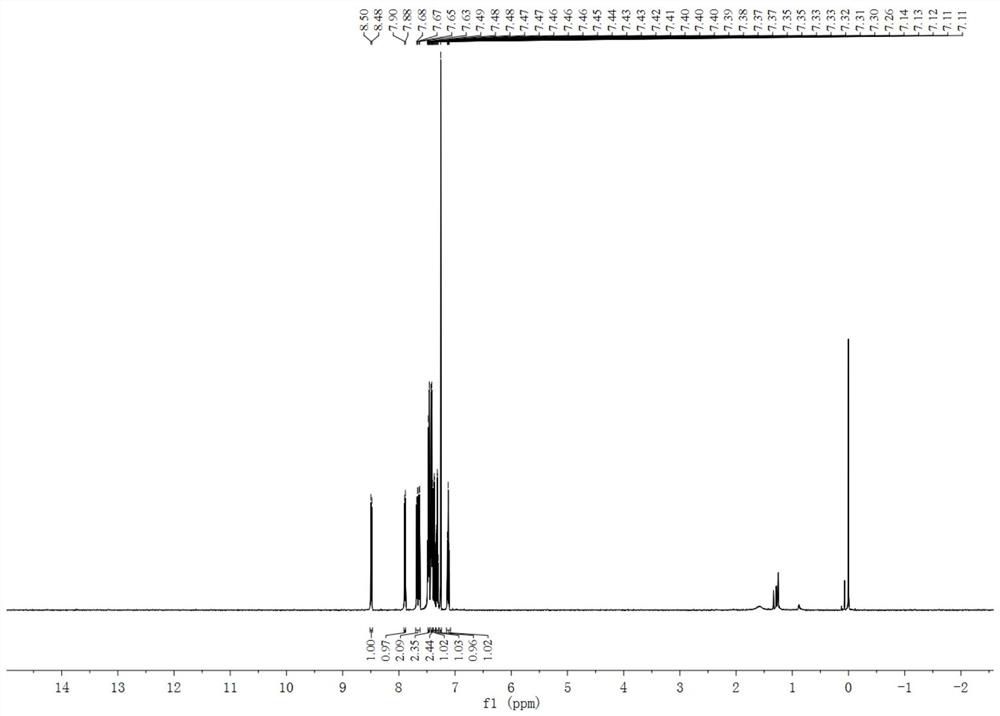
[0032] Under the condition of 66°C, 1.0mmol of 9-fluorenone oxime and 1.2mmol of 2-(trimethylsilyl)phenyltrifluoromethanesulfonate and 2.0mmol of cesium fluoride as base and 2mmol of 18-crown -6-ether was used as a phase transfer catalyst and reacted in 2 mL of anhydrous toluene solvent for 28 hours to obtain the crude product of the triphenylene compound; the crude product of the prepared triphenylene compound was washed with water, extracted with ethyl acetate, and spin-dried under reduced pressure, The volume ratio of ethyl acetate:petroleum ether=1:60 was separa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com